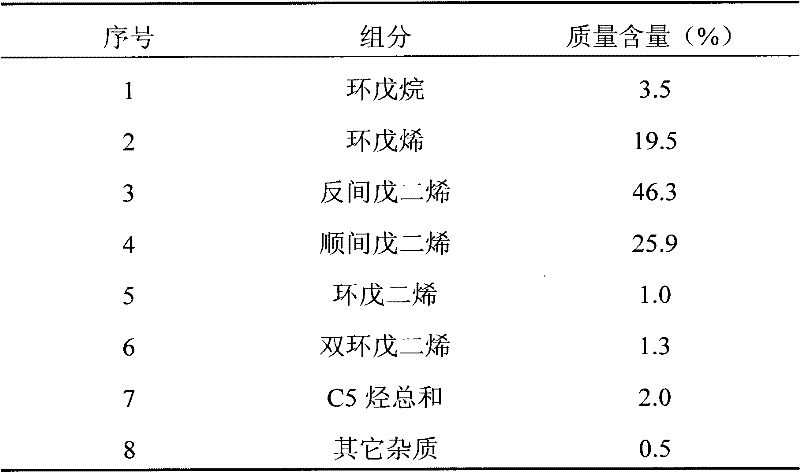
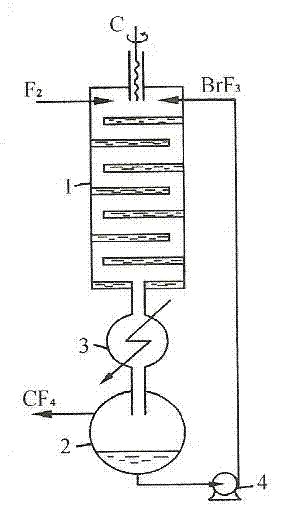
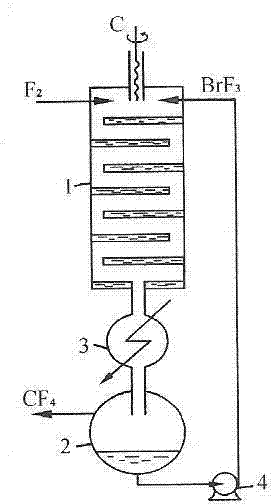
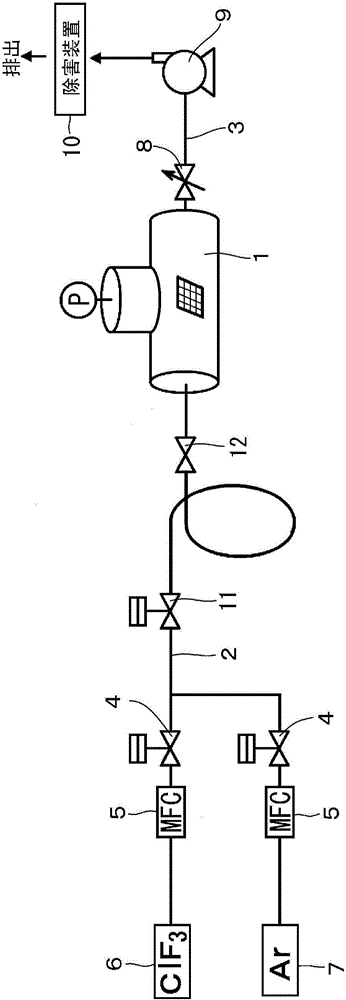
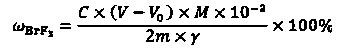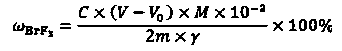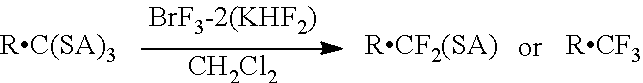Patents
Literature
41 results about "Bromine trifluoride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
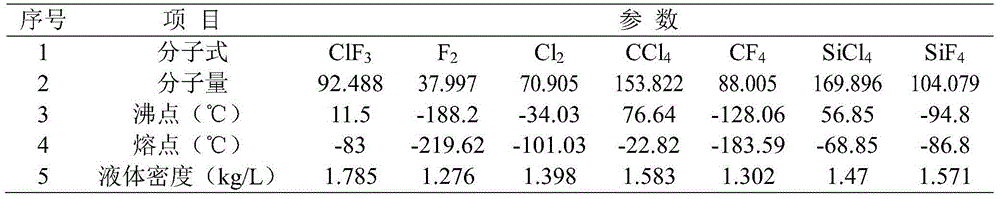
Bromine trifluoride is an interhalogen compound with the formula BrF₃. It is a straw-coloured liquid with a pungent odor. It is soluble in sulfuric acid but reacts violently with water and organic compounds. It is a powerful fluorinating agent and an ionizing inorganic solvent. It is used to produce uranium hexafluoride (UF₆) in the processing and reprocessing of nuclear fuel.
Concentrated aqueous bromine solutions and their preparation
Described is a process of producing a concentrated liquid biocide formulation. Mixed together are (a) bromine chloride or bromine and (b) an aqueous solution of alkali metal salt of sulfamic acid having a pH of at least about 7, in amounts such that (i) the active bromine content of the solution is at least about 100,000 ppm (wt / wt), and (ii) the atom ratio of nitrogen to active bromine from (a) and (b) is greater than 1 when bromine is used and is greater than 0.93 when bromine chloride is used. Use of bromine chloride as the source of the active bromine in the process is preferred because in the resulting aqueous compositions, all of the bromine of the bromine chloride is made available as active bromine in solution. In other words, the chlorine of the bromine chloride is converted in the process to dissolved alkali metal chloride salt, thereby liberating all of the bromine as the active bromine content of the biocidal composition.
Owner:ALBEMARLE CORP
Method for deactivating and recovering boron trifluoride when producing polyisobutenes
InactiveUS6939943B2Speed up the processCatalyst regeneration/reactivationAlcoholCationic polymerization
The invention relates to a method for deactivating and recovering boron trifluoride when producing polyisobutenes by means of cationic polymerization of isobutene or hydrocarbon streams containing isobutene in the liquid phase in the presence of boron trifluoride or in the form of a boron trifluoride catalyst complex. The catalyst complex is separated, essentially in the liquid phase, from the reactor discharge. The method comprises the following steps: a) removing from the polymerization reactor at −60 to 020 C., methanol, ethanol or a mixture of methanol and ethanol in such a quantity that an alcohol phase rich in boron trifluoride is formed; b) separating the alcohol phase according to (a) and, (c) optionally recycling the boron trifluoride of the alcohol phase obtained from (b) to the method in a suitable manner.
Owner:BASF AG
Concentrated aqueous bromine solutions and their preparation and use
InactiveUS6652889B2Improve solubilityMinimize extentBiocideSpecific water treatment objectivesChloride saltNitrogen
Described is a process of producing a concentrated liquid biocide formulation. Mixed together are (a) bromine chloride or bromine and (b) an aqueous solution of alkali metal salt of sulfamic acid having a pH of at least about 7, in amounts such that (i) the active bromine content of the solution is at least about 100,000 ppm (wt / wt), and (ii) the atom ratio of nitrogen to active bromine from (a) and (b) is greater than 1 when bromine is used and is greater than 0.93 when bromine chloride is used. Use of bromine chloride as the source of the active bromine in the process is preferred because in the resulting aqueous compositions, all of the bromine of the bromine chloride is made available as active bromine in solution. In other words, the chlorine of the bromine chloride is converted in the process to dissolved alkali metal chloride salt, thereby liberating all of the bromine in the biocidal composition as active bromine capable of providing biocidal activity.
Owner:ALBEMARLE CORP
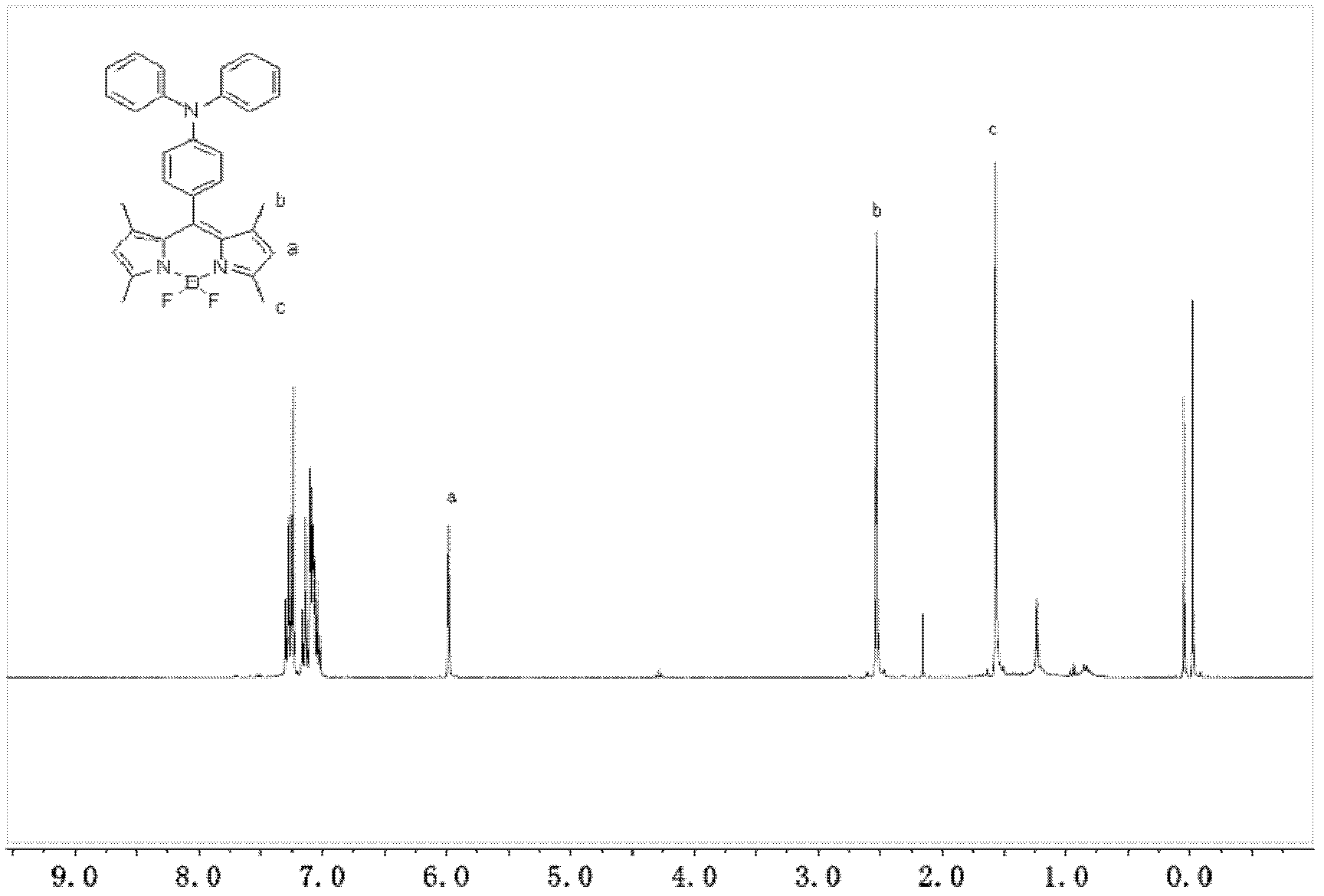
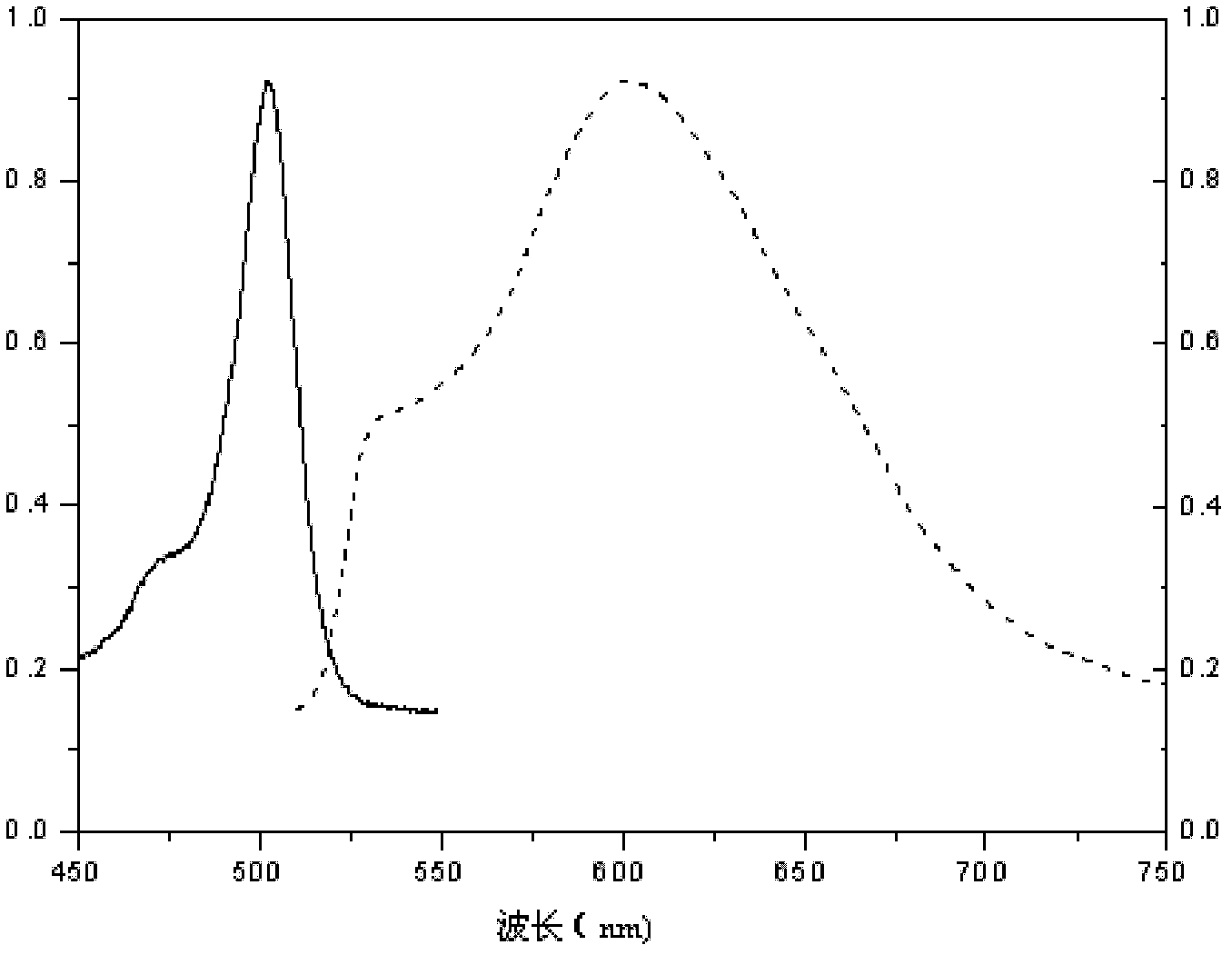
1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound and preparation method thereof
InactiveCN102321109AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMethylene DichlorideOrganic synthesis
The invention relates to a 1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound and a preparation method thereof, which belong to the technical field of organic synthesis. The existing photocatalysts have few varieties and low conversion rate, and the existing bodipy dye has low infrared fluorescent efficiency and small Stokes displacement. The invention provides the 1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound. The preparation method comprises the steps that: 4-formoxyl tetramethyl and 2,4-dimethyl pyrrole are dissolved in organic solvents, trifluoroacetic acid or monoprop is used as catalysts, and a reaction system is formed; 2,3-dichloro-5,6-dicyan-1,4-para-benzoquinone with the same mol ratio as the 4-formoxyl tetramethyl is taken, is dissolved in the methylene dichloride and is added into the reaction system; and one of triethylamine, triisopropyl amine and N,N-diisopropylethylamine is added into the reaction system, boron trifluoride etherate is added under the ice bath, and final products are generated.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Preparation method of chlorine trifluoride
ActiveCN104477849AStable reaction temperatureTimely supplementInter-halogen compoundsFine chemicalCorrosion
The invention discloses a preparation method of chlorine trifluoride and belongs to the field of fine chemical engineering. The method comprises the following steps: carrying out a bubbling reaction on liquid-phase chloride and fluorine gas at a certain temperature to obtain a product 1; then charging the product 1, a diluent gas and fluorine gas in a synthesis reactor, and reacting at a certain temperature to obtain a product 2; finally carrying out liquidation, lightweight material discharge and vaporization treatments on the product 2 to obtain chlorine trifluoride. The raw materials in the preparation method are low in price and easily available, thus avoiding the adoption of chlorine with high corrosion and high toxicity, improving safety during production, transportation and storage processes; moreover, the reactor is simple in structure and convenient for material charging and discharging; the reaction conversion rate is high; by-products can be easily separated; and the preparation method can be put into industrial production easily.
Owner:PERIC SPECIAL GASES CO LTD
Preparation method and device of chlorine trifluoride
ActiveCN104477850ASimple production processAvoid corrosionInter-halogen compoundsBoron trifluorideReaction temperature
The invention discloses a preparation method and device of chlorine trifluoride and belongs to the field of fine chemical engineering. The method comprises the following steps: mixing chlorine gas, fluorine gas and a diluent gas, and then charging the mixed gas in a reactor filled with a catalyst, and reacting at 100-400 DEG C to obtain a product 1; then carrying out cooling, liquidation, lightweight material discharge and vaporization treatments on the product 1 to obtain the chlorine trifluoride. The device comprises a catalytic reactor, a low-temperature collector, a chlorine trifluoride storage tank and a vacuum pump, wherein the catalytic reactor, the low-temperature collector and the chlorine trifluoride storage tank are sequentially connected through pipelines. The process is low in reaction temperature, short in reaction time and high in product yield; the device has the advantages that the length of the reactor is greatly shortened and the production process of chlorine trifluoride is simplified.
Owner:PERIC SPECIAL GASES CO LTD
Method for preparing graphite fluoride at low temperature
The invention discloses a method for preparing graphite fluoride at low temperature. The method comprises the following steps: using ferric fluoride as a catalyst, and performing reaction of crystalline flake graphite and bromine trifluoride at low temperature to generate a graphite intercalation compound; then, performing reaction of potassium manganese hexafluoride and antimony pentafluoride to generate fluorine; and performing reaction of the fluorine generated and the graphite intercalation compound to form the graphite fluoride with high fluorine content. Through purifying treatment by a solvent, the prepared graphite fluoride is high in purity.
Owner:东莞市致格电池科技有限公司
Device and method for purifying bromine trifluoride
InactiveCN107311110AImprove separation efficiencyEasy to separateInter-halogen compoundsBromine trifluoridePre treatment
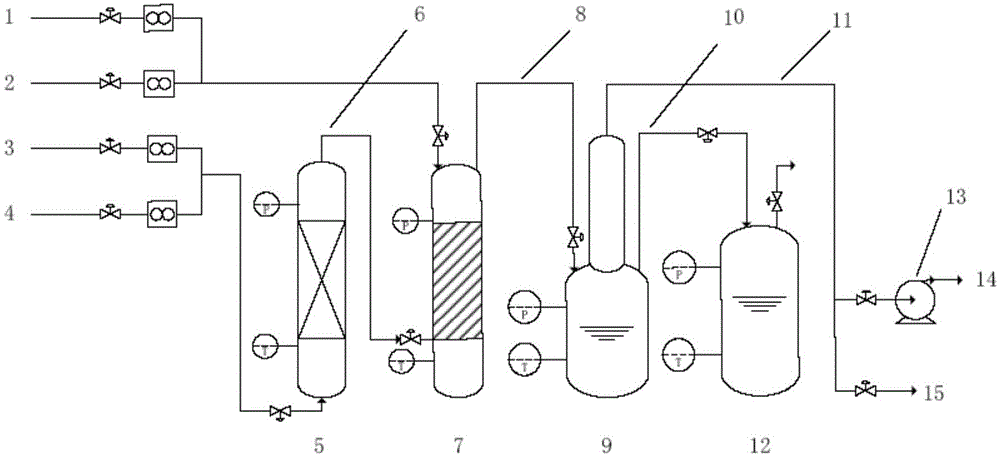
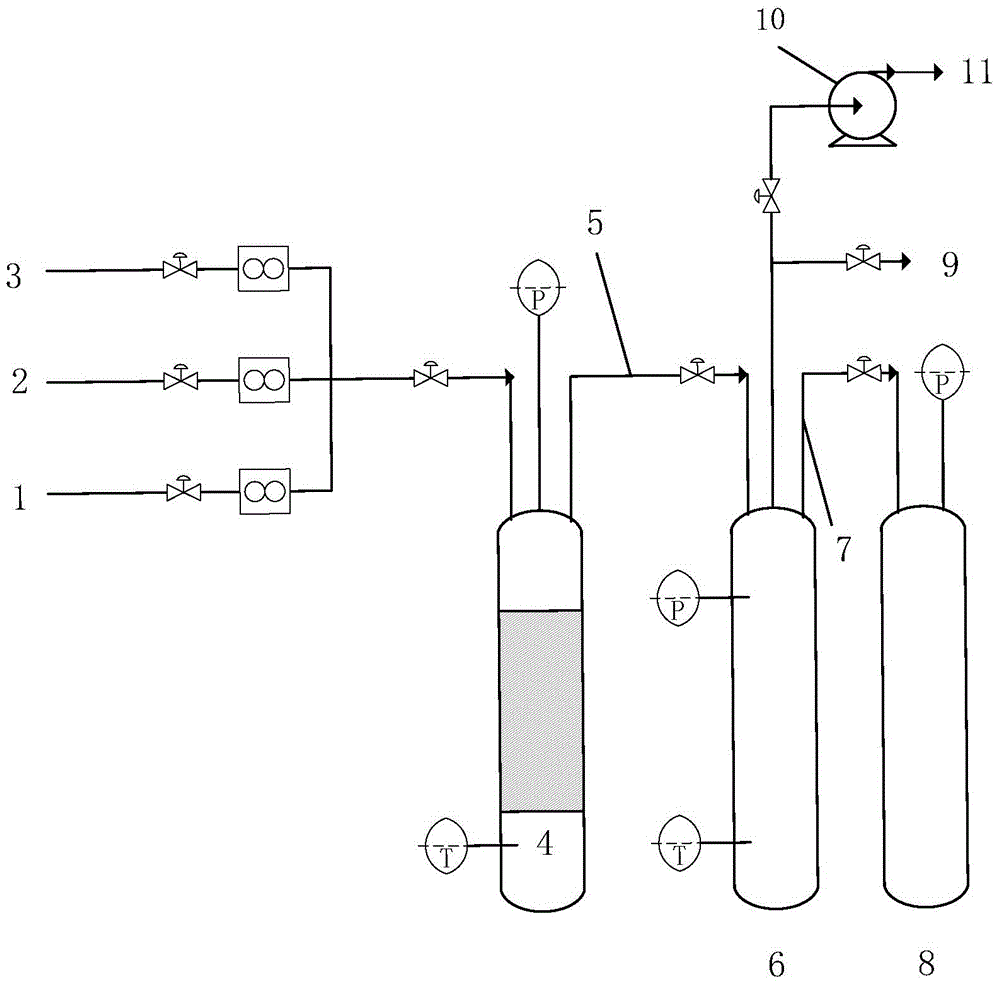
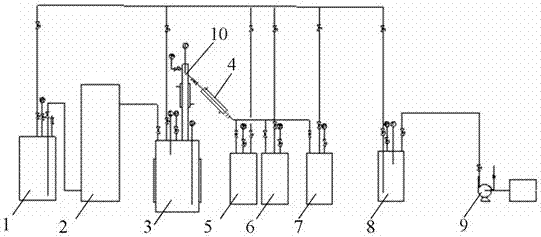
The invention discloses a device and a method for purifying bromine trifluoride. The device comprises a vaporizer, an adsorption tower, a rectification purification device, a condensation collecting pipe, a front fraction collecting device, a main fraction collecting device, a rear fraction collecting device, a tail gas recycling system and a vacuum pump. The method includes steps of (i) pretreatment, (ii) adsorption purification and (iii) intermittent rectification purification. The device and the method for purifying the bromine trifluoride by means of adsorption and then intermittent rectification have the advantages that the device and the method are high in phase separation efficiency, light and heavy components can be thoroughly separated from one another, bromine trifluoride products obtained by the aid of the device and the method are high in purity, the purity of the bromine trifluoride products can reach 99.5%, and the device and the method are high in production efficiency.
Owner:天津长芦华信化工股份有限公司
Method for catalyzed preparation of C5 petroleum resin by utilizing boron trifluoride
The invention relates to a method for catalyzed preparation of C5 petroleum resin by utilizing boron trifluoride. The method comprises the steps of rectifying C5 fractions to obtain a polymerization raw material A with a boiling point of 20-70 DEG C, causing enriched materials of styrene, alpha-methyl styrene and vinyl toluene to serve as a polymerization raw material B, evenly mixing the raw material A, the raw material B and saturated varsol according to a mass ratio of 1:(0-0.5):(1-1.5) to obtain ingredients; reacting for 2-5 h at 50-170 DEG C under the effect of a Friedel-Crafts catalyst of a BF3 system, and obtaining polymerization liquid; and obtaining the modified C5 petroleum resin through solvent removal and oligomer removal. The method is simple and convenient in process, the adaptability to complex C5 raw materials is good, and the C5 petroleum resin with a high softening point can be prepared.
Owner:NINGBO YONGHUA RESIN +1
Process for preparing boron trifluoride gas
InactiveCN102249256ALow reaction temperatureImprove securityBoron halogen compoundsHydrogen fluorideAbsorption column
The invention relates to a process for preparing boron trifluoride gas. The process comprises the following steps of: a, mixing, and generating gas, namely mixing boric acid and 20 percent fuming sulphuric acid under normal pressure, mixing the mixed acid of boric acid and fuming sulphuric acid, and hydrogen fluoride gas in a reaction tower, contacting and reacting in the mass ratio of the mixed acid of boric acid and fuming sulphuric acid to the hydrogen fluoride gas of 9:(0.8-0.9) to generate fluoboric acid, and performing decomposition reaction of fluoboric acid at the presence of the fuming sulphuric acid at the temperature of between 80 and 120 DEG C to generate boron trifluoride gas; b, reacting in an absorption column, namely drying and purifying the boron trifluoride gas and byproduct sulfuric acid which are produced in the gas making process; and c, utilizing the byproduct sulfuric acid, namely adding sodium sulfate into a reaction kettle, adding the byproduct sulfuric acid, and reacting in the mass ratio of sodium sulfate to byproduct sulfuric acid of 1:(4-6), and reacting at normal temperature to generate sodium acid sulfate. The process has the advantages of low reaction temperature, high safety, no waste residue or waste gas, and environment-friendliness, and the waste liquor can be recycled.
Owner:润泰化学南通有限公司
Preparation method of cefazolin sodium intermediate TDA (tolylenediamine)
InactiveCN102633813AAvoid pollutionReduce manufacturing costOrganic chemistrySodium bicarbonateFiltration
The invention provides a preparation method of a cefazolin sodium intermediate TDA (tolylenediamine), which comprises the following steps: by using acetone, acetonitrile, aether, dimethyl carbonate, dichloromethane or chloroform as a solvent, filling 7-ACA (7-aminocephalosporanic acid) and thia-diazole in a weight ratio of 1:1.2 into a reaction vessel, introducing boron trifluoride gas into the reaction vessel, reacting for 1-8 hours while controlling the temperature in the reaction vessel at 0-50 DEG C until the residual 7-ACA is less than 1%, hydrolyzing and crystallizing the reaction product by using sodium carbonate, sodium bicarbonate or ammonia water, carrying out vacuum filtration, washing, and drying to obtain the finished TDA product. The invention does not use boron trifluoride solid complex in the preparation process, and therefore, does not generate waste liquor containing boron trifluoride, thereby preventing the boron trifluoride solid complex from generating pollution and abundant waste liquor to be recovered in the preparation process. Besides, the invention prevents operating personnel from directly contacting the boron trifluoride solid complex in the whole preparation process, thereby lowering the safety risk in the use process.
Owner:HEILONGJIANG HAOYUN FINE CHEM
Detoxifying method of chlorine trifluoride
ActiveCN102143793AFluorine reductionGas treatmentDispersed particle separationHalogenBoron trifluoride
The invention discloses a detoxifying method which is a method for eliminating unwanted substances from a mixed gas composed of at least chlorine trifluoride and fluorine with a wet scrubber. As a pre-treatment step for the elimination of unwanted substances with the wet scrubber, the method involves a step of adding a halogen gas (X2) (wherein X represents Cl, Br or I) to the mixed gas to cause the reaction between fluorine contained in the mixed gas with the halogen gas (X2) (wherein X represents Cl, Br or I), thereby reducing the content of fluorine in the mixed gas and preventing the generation of perchloryl fluoride (ClO3F) in the wet scrubber.
Owner:CENT GLASS CO LTD
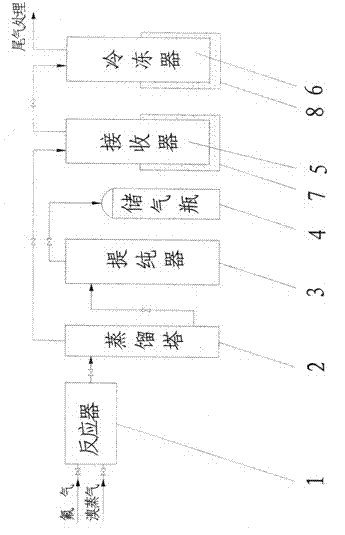
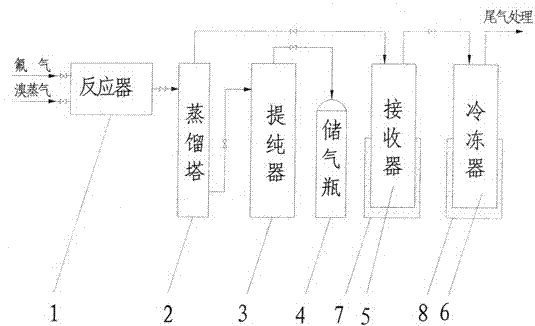
Method for preparing bromine trifluoride
ActiveCN102502503AReduce pollutionEasy to operateInter-halogen compoundsReaction temperaturePhysical chemistry
The invention discloses a method for preparing bromine trifluoride. The method comprises the following steps of: (i) pre-treating a reactor, pre-heating the reactor to 100-120 DEG C, and pumping air out of the reactor till the pressure is about -0.095 MPa; (ii) carrying out synthetic reaction on fluorine gas and bromine vapor at a reaction temperature of 120-160 DEG C so as to obtain a crude product of bromine trifluoride; (iii) removing light-component impurities such as bromine pentafluoride and bromine elementary substance out of the crude product of bromine trifluoride; (iv) purifying bromine trifluoride; and (v) carrying out tail gas treatment. The method for preparing bromine trifluoride disclosed by the invention has the advantages of simple equipment, convenience for operation, safe equipment operation and little environment pollution. Furthermore, the purity of the product can be more than 98%.
Owner:RES INST OF PHYSICAL & CHEM ENG OF NUCLEAR IND
Preparation method of boron trifluoride tetrahydrofuran
InactiveCN102911195ALow costThe reaction temperature is easy to controlGroup 3/13 element organic compoundsBoron trifluorideReaction temperature
The invention discloses a preparation method of boron trifluoride tetrahydrofuran, which is characterized in that the boron trifluoride tetrahydrofuran is prepared by tetrahydrofuran vapour and boron trifluoride gas through complex reaction. The preparation method has the advantages that the boron trifluoride tetrahydrofuran is prepared by the tetrahydrofuran vapour and the boron trifluoride gas through the complex reaction, the cost is low and the reaction temperature is easy to control at a room or higher temperature.
Owner:江峰
Preparation method of lithium tetrafluoroborate
InactiveCN109264736AQuality improvementSimple processSecondary cellsTretrafluoboric acidHigh energyFiltration
The invention relates to a preparation method of lithium tetrafluoroborate. According to the technical scheme, the preparation method comprises the following steps that 1, under the ambient temperature and pressure, lithium fluoride is added into an esters solvent to obtain a lithium fluoride turbid liquid; 2, boron trifluoride gas is introduced into the lithium fluoride turbid liquid, and reaction is carried out for 1-2 h to obtain a lithium tetrafluoroborate solution; 3, unreacted trace lithium fluoride of the solution obtained in step 2 is filtered through a microporus filter; 4, the liquidafter filtration in step 3 is completed is subjected to concentration and degassing; 5, the solution after concentration is completed in step 4 is subjected to cooling crystallization, filtration anddrying to obtain a lithium tetrafluoroborate finished product. The preparation method of the lithium tetrafluoroborate has the advantages that adopted boron trifluoride is an industrial grade raw material, and is low and easy to obtain; the lithium fluoride forms the turbid liquid in the esters solvent, so that reaction is easily carried out, the operation is simple and convenient, the safety isimproved, and the problems of the risk in reaction and preparation when hydrogen fluoride is adopted as a solvent, excessive amount of free acid of the product, high energy consumption of ultralow-temperature crystallization and the like are avoided.
Owner:东营石大胜华新能源有限公司
Low temperature preparation method of boron trifluoride dimethyl carbonate complex compound
InactiveCN103044474AReduce reaction energy consumptionLow reaction temperatureGroup 3/13 element organic compoundsRaw materialCrystal
The invention belongs to a chemical engineering product, a boron trifluoride dimethyl carbonate complex compound, in particular relates to a low temperature preparation method of the boron trifluoride dimethyl carbonate complex compound. The preparation method comprises the following steps: in a gas generating container, using boric acid and anhydrous hydrofluoric acid as raw materials, dehydrating and generating boron trifluoride gas, and sulfur trioxide is used as an dehydrating agent; in a purification container, purifying the boron trifluoride gas generated through concentrated sulphuric acid; in a complexing container, performing a complexing reaction of the purified boron trifluoride gas and the dimethyl carbonate; and in a filter, cooling down and crystallizing the liquid reactant of the complexing reaction, and the solid gathered is the boron trifluoride dimethyl carbonate complex compound. The technical scheme provided by the invention enables the temperature of the gas generating reaction and complexing reaction to be close to the room temperature, decreases energy consumption during production process and is low-carbon and environment friendly; the production process is safer and more controllable; and the boron trifluoride dimethyl carbonate complex compound is a solid white crystal, wherein the content of boron trifluoride is 40-43 percent in weight and the property of the boron trifluoride is stable.
Owner:SHANDONG HEYI GAS CO LTD DONGYING CITY +1
Preparation method for pentadiene petroleum resin
The invention relates to a preparation method for pentadiene petroleum resin. The method comprises the following step of: synthesizing pentadiene petroleum resin by taking a pentadiene concentrate as a raw material and taking a boron trifluoride complex as a catalyst, wherein the catalyst consists of boron trifluoride and a complexing agent; the complexing agent consists of C1-C10 alcohol and benzene alkyl ether C6H5-OR; in the formula, R is methyl, ethyl or butyl; the molar ratio of the C1-C10 alcohol to the benzene alkyl ether is 2-8; the molar ratio of the boron triflouride to the complexing agent is 0.5-0.8; and a preparation method for the boron triflouride complex serving as the catalyst comprises the following steps of: adding the C1-C10 alcohol and the benzene alkyl ether into a reactor; cooling the materials in the reactor to 0 DEG C to 30 DEG C below zero while stirring; introducing boron triflouride gas into a container within 2-6 hours; and controlling the reaction temperature at -15+ / -5 DEG C. The color phase (Fe-Co) of the pentadiene petroleum resin produced with the method is less than or equal to 5, and the softening point is 89 DEG C.
Owner:PETROCHINA CO LTD
Method for directly preparing tetrafluoromethane from fluorocarbon using explosion suppressant
InactiveCN102516019AContinuous and stable productionSolve the disadvantages of unstable purificationHalogenated hydrocarbon preparationHalogenProcess engineering
The invention discloses a method for directly preparing tetrafluoromethane by a fluorocarbon direct synthesis process using bromine trifluoride as an explosion suppressant, is a process for preparing high-purity tetrafluoromethane by the reaction of 60-mesh petroleum coke and F2 under a condition that fluorinated halogen is used as an explosion suppressant, wherein the ratio of the added amount of the explosion suppressant to the volume of fluoride is 1: 20; the added amount of the petroleum coke is 500-550 kg every time, and the added amount of the F2 is 10-12 L / min; and the high-purity tetrafluoromethane is obtained at last after port-treatment. The preparation method disclosed by the invention is reasonable in process and simple in production, as well as is the most simple and ideal technology for preparing tetrafluoromethane.
Owner:TIANJIN TAIHENG GASES
Method for treating inner surface of chlorine trifluoride supply path in device using chlorine trifluoride
ActiveCN104040699AReduce concentrationForm evenlySemiconductor/solid-state device manufacturingChemical vapor deposition coatingBromine trifluorideFluoride
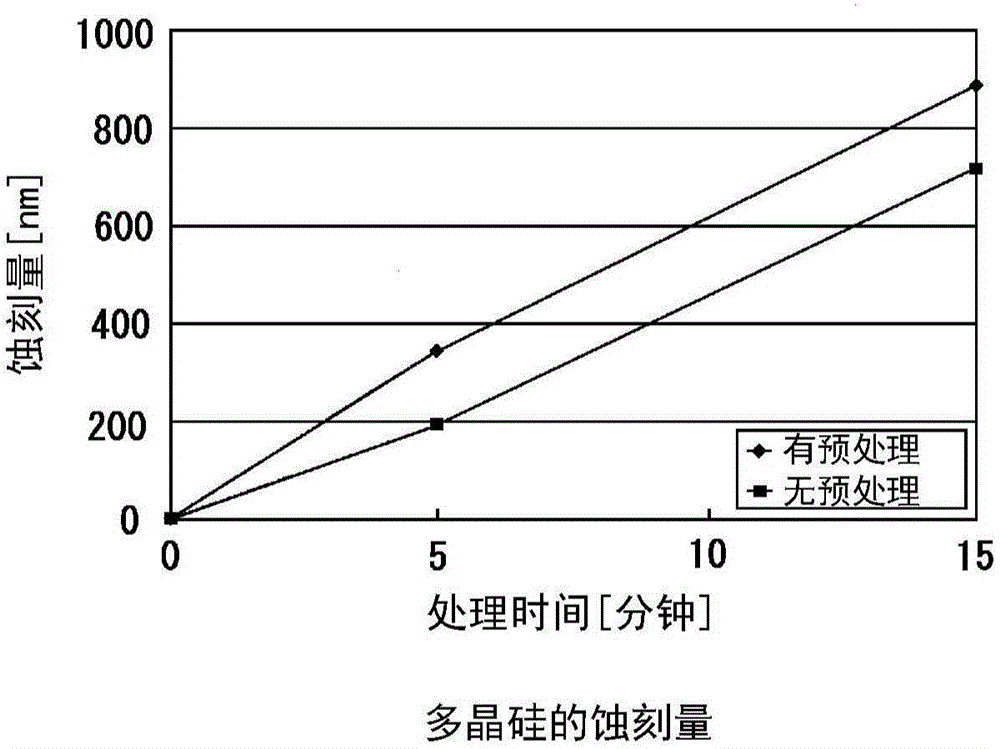
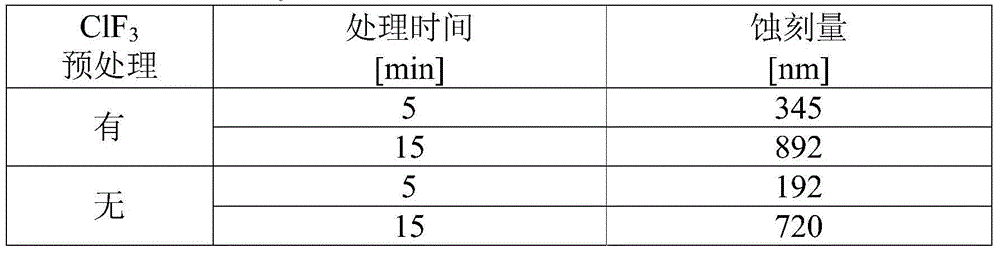
Provided is a method for treating the inner surface of a chlorine trifluoride supply path that enables reliable suppression of drops in the concentration of ClF3 in a reaction chamber during treatment work. A gas supply path (2) and a gas discharge path (3) are integrally connected to a treatment chamber (1) of a treatment device in which chlorine trifluoride is used as an etching gas. Chlorine trifluoride gas with a concentration equal to or greater than the concentration of the chlorine trifluoride gas supplied during etching treatment operation is allowed to act on the inner surfaces of at least the treatment chamber (1) and the gas supply path (2) from among the treatment chamber (1), the gas supply path (2), and the gas discharge path (3), thus coating the inner surfaces of at least the treatment chamber (1) and the gas supply path (2) with a fluoride film.
Owner:IWATANI CORP
Production technological method for boron trifluoride acetonitrile complex solid mixture
InactiveCN108864163AIncrease productionAdd process stepsGroup 3/13 element organic compoundsEnvironmental resistancePollution
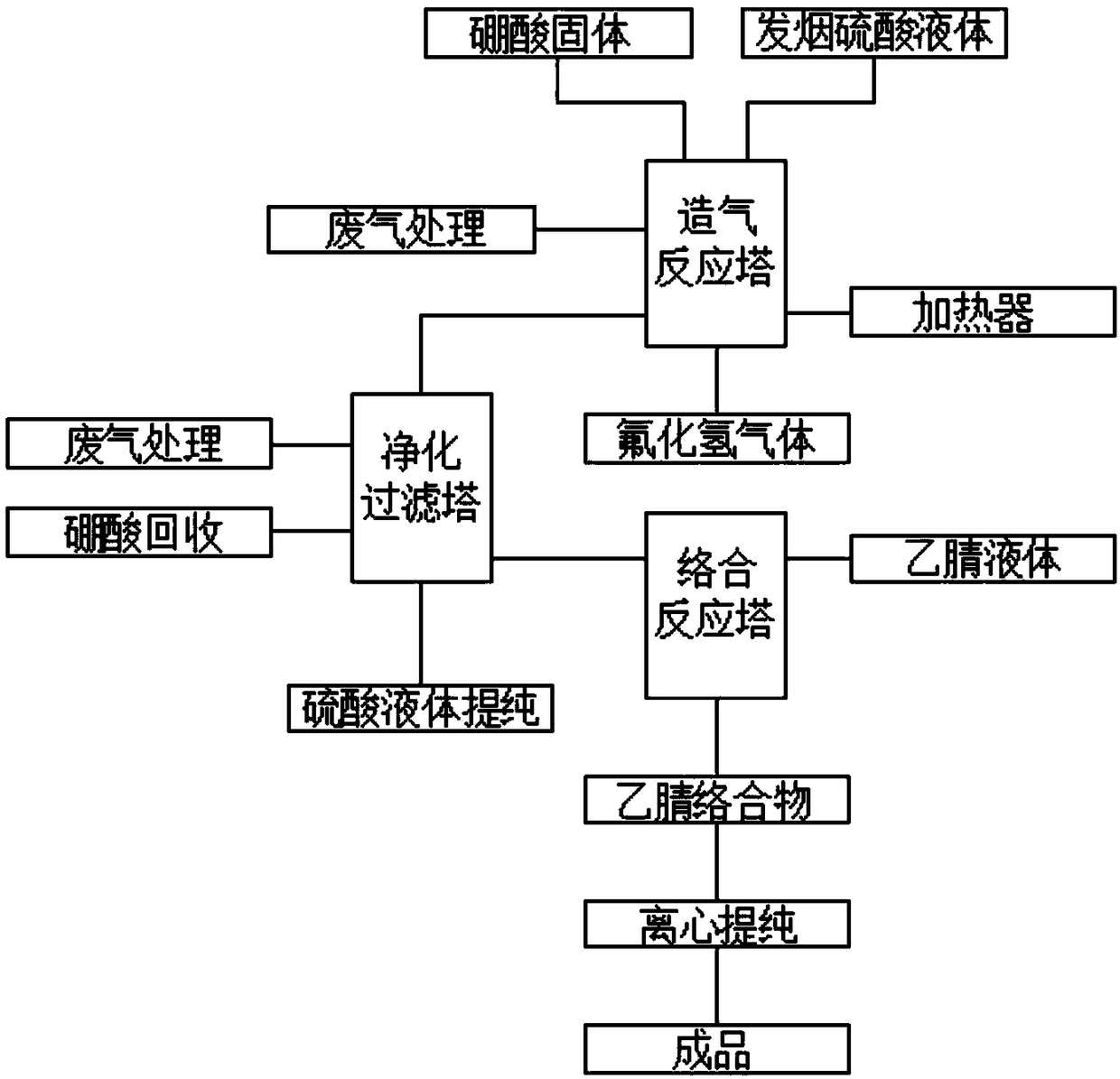
The invention belongs to the field of chemical technologies, and particularly relates to a production technological method for a boron trifluoride acetonitrile complex solid mixture. The method aims at solving the problems that the existing production process flow is relatively simple, the yield of finished products is relatively small, the resource waste is easily caused, in addition, the waste gas treatment link and the solid recovery link do not exist in the production process, the pollution to the environment is caused, the environmental protection purpose is not achieved, and the like. According to the method, boric acid solid and fuming sulfuric acid liquid are uniformly mixed, a gas-making reaction tower is preheated for 10 minutes through a heater, the temperature is controlled tobe within 200-300 DEG C, mixed acid of the boric acid solid and the fuming sulfuric acid liquid is added into the gas-making reaction tower from the top, and meanwhile, hydrogen fluoride gas is compressed and then is added into the gas making reaction tower from the bottom. The method has the advantages that the yield of the finished products is increased, the resource waste is avoided, the wastegas treatment link and the solid recovery link are increased, the pollution to the environment is avoided, and the environmental protection purpose is achieved.
Owner:珠海市格特生物科技有限公司
Production method of high purity silver tetrafluoroborate
InactiveUS8501138B2High purityPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesBoron halidesHydrofluoric acidSilver tetrafluoroborate
A production method of high purity silver tetrafluoroborate, capable of producing silver tetrafluoroborate (AgBF4) at purity higher than the conventional, without using an organic solvent. The production method of the present invention is characterized in that the method comprises the step of: reacting silver fluoride with boron trifluoride in the presence of anhydrous hydrofluoric acid. Boron trifluoride is delivered into a solution obtained by dissolving or suspending silver fluoride in an anhydrous hydrofluoric acid solution.
Owner:STELLA CHEMIFA CORP
Preparation of boron trifluoride and sulfuric acid from boron trifluoride hydrate
InactiveCN1121270CPhysical/chemical process catalystsFatty/oily/floating substances removal devicesBoron trifluorideTrifluoride
Boron trifluoride (BF3) and sulfuric acid are prepared by reacting oleum with a technical BF3 hydrate to produce BF3 gas, which is recovered, and sulfuric acid which is treated with hydrogen peroxide and then with air.
Owner:ELF ATOCHEM SA
Detection method of purity of bromine trifluoride product
InactiveCN108445148ALow costEasy to operateChemical analysis using titrationMaterial analysis by observing effect on chemical indicatorBromine trifluorideHydrolysis
The invention discloses a detection method of purity of a bromine trifluoride product. The detection method comprises the following steps: (I) pretreating the product; (II) extracting product hydrolysis liquid; (III) analyzing the purity of the bromine trifluoride product; and (IV) calculating the purity of the bromine trifluoride product. The detection method of the purity of the bromine trifluoride product is simple and practicable, is convenient to operate, achieves low cost, high safety and high measurement accuracy, and is especially applicable to analysis of the bromine trifluoride product which is prepared from fluorine and bromine through direct reaction, and is low in content of bromine pentafluoride in impurities after the bromine trifluoride product is fully distilled.
Owner:天津长芦华信化工股份有限公司
A kind of preparation method and device of chlorine trifluoride
ActiveCN104477850BSimple production processAvoid corrosionInter-halogen compoundsReaction temperatureVaporization
The invention discloses a preparation method and device of chlorine trifluoride and belongs to the field of fine chemical engineering. The method comprises the following steps: mixing chlorine gas, fluorine gas and a diluent gas, and then charging the mixed gas in a reactor filled with a catalyst, and reacting at 100-400 DEG C to obtain a product 1; then carrying out cooling, liquidation, lightweight material discharge and vaporization treatments on the product 1 to obtain the chlorine trifluoride. The device comprises a catalytic reactor, a low-temperature collector, a chlorine trifluoride storage tank and a vacuum pump, wherein the catalytic reactor, the low-temperature collector and the chlorine trifluoride storage tank are sequentially connected through pipelines. The process is low in reaction temperature, short in reaction time and high in product yield; the device has the advantages that the length of the reactor is greatly shortened and the production process of chlorine trifluoride is simplified.
Owner:PERIC SPECIAL GASES CO LTD
Preparation method of boron trifluoride complex
InactiveCN101525343BAvoid pollutionLow reaction temperatureOrganic-compounds/hydrides/coordination-complexes catalystsGroup 3/13 element organic compoundsSlagReaction temperature
The invention relates to a catalyst applied to the manufacturing technique of cephalo-type products, comprising the steps of firstly adding sulphuric acid or fuming acid in a reaction kettle, and then adding boron anhydrous or boric acid, infusing sulphur trioxide in the reaction kettle, and finally infusing hydrogen fluoride in the reaction kettle, the contents of the components are respectively2-5 parts of sulphuric acid or 3-5 parts of fuming acid, 0.4-1.5 parts of boron anhydrous or 0.4-1.5 parts of boric acid, 2-4 parts of sulphur trioxide and 2-7 parts of hydrogen fluoride. For the production of boron trifluoride-dimethyl carbonate complex, the raw materials comprise 1.25-1.5 parts of boron trifluoride and 5-10 parts of dimethyl carbonate, and solid boron-trifluoride dimethyl carbonate complex is separated by centrifuging and then packed into a transportation bag, and finally returning the mother liquor to a complexing kettle to carry out complexation again. For the production of boron trifluoride-acetonitrile complex, the raw materials comprise 0.8-1 part of boron trifluoride and 4-5 parts of acetonitrile, and the raw materials are directly put into a package bucket. The invention has the advantages of low reaction temperature, moderate reaction and high safety. The invention does not generate waste slag and waste liquor, avoids environmental pollution and saves energysource. The product of the invention is stable and has high yield. The method is simply operated and suitable for mass production.
Owner:于志红
Method for preparing boron-10 acid with boron trifluoride-10
The invention relates to a method for preparing boron-10 acid with boron-10 trifluoride. The method includes following steps: carrying out a reaction between the boron-10 trifluoride and calcium methoxide to obtain trimethyl borate-10 with absolute methanol being a solvent; carrying out a solid-liquid separation process to a reaction product; performing hydrolysis to a liquid mixture to obtain the boron-10 acid. The method is divided into two processes: an esterification process and a hydrolysis process, wherein methanol is used as the solvent and a reaction is carried out between the boron-10 trifluoride and the calcium methoxide to obtain the mixture composed of the trimethyl borate-10 and the methanol in the esterification process. The reaction product is subjected to a distillation process with a distillate being subjected to hydrolysis to obtain a mixed solution composed of boric acid, methanol and water. A condensation process and a crystallization process to obtain the boron-10 acid. A solid by-product obtained after the distillation is single in content, wherein a main component in the solid by-product is calcium fluoride. A yield of the boron-10 acid is more than 95.2% through the method and a purity of the boron-10 acid is more than 99.7% after the boron-10 acid being detected through a detection method in GBT12684-2006.
Owner:TIANJIN UNIV
Fluorinating agent
ActiveUS20160332877A1Improve responseReduced responseOrganic compound preparationCarboxylic acid esters preparationHydrogenBromine trifluoride
An object of the present invention is to provide a novel substance that has a high reactivity as a fluorinating agent, is effectively used in various fluorination reactions, and is safely handled even in air. As the solution for achieving this object, the present invention provides a complex obtained by reacting bromine trifluoride with at least one metal halide selected from the group consisting of halogenated metals and halogenated hydrogen metals in a nonpolar solvent. This complex serves as a fluorinating agent that provides excellent fluorination performance and that is stable in air.
Owner:DAIKIN IND LTD +1
Method for co-production of propylene glycol during production of boron trifluoride
ActiveCN104355315AHigh purityReduce corrosionBoron halogen compoundsPreparation by hydrolysisImpurityCorrosion
The invention discloses a method for the co-production of propylene glycol during the production of boron trifluoride. Boric acid, hydrofluoric acid, water and propylene oxide are used as raw materials, and the method comprises the following steps of hydrolyzing the propylene oxide in the presence of boron trifluoride, performing ring opening reaction on the propylene oxide to generate propylene glycol, and preparing boron trifluoride at the same time. According to the method, the adoption of sulfuric acid as a reaction raw material is avoided, so that corrosion to production equipment is reduced, and the environmental pollution is avoided; the prepared boron trifluoride does not contain impurities such as HF, SO3 and SO2, so that the purity of the boron trifluoride is obviously improved; the problem of existence of waste sulfuric acid difficult to treat in a byproduct produced by the production of boron trifluoride is solved, and the technical problem of serious corrosion to the production equipment is solved; propylene glycol is co-produced during the production of the boron trifluoride, and can be used for production after being rectified, so that the production cost is greatly lowered, and the utilization rate of the byproduct is increased.
Owner:SHANDONG HEYI GAS CO LTD DONGYING CITY
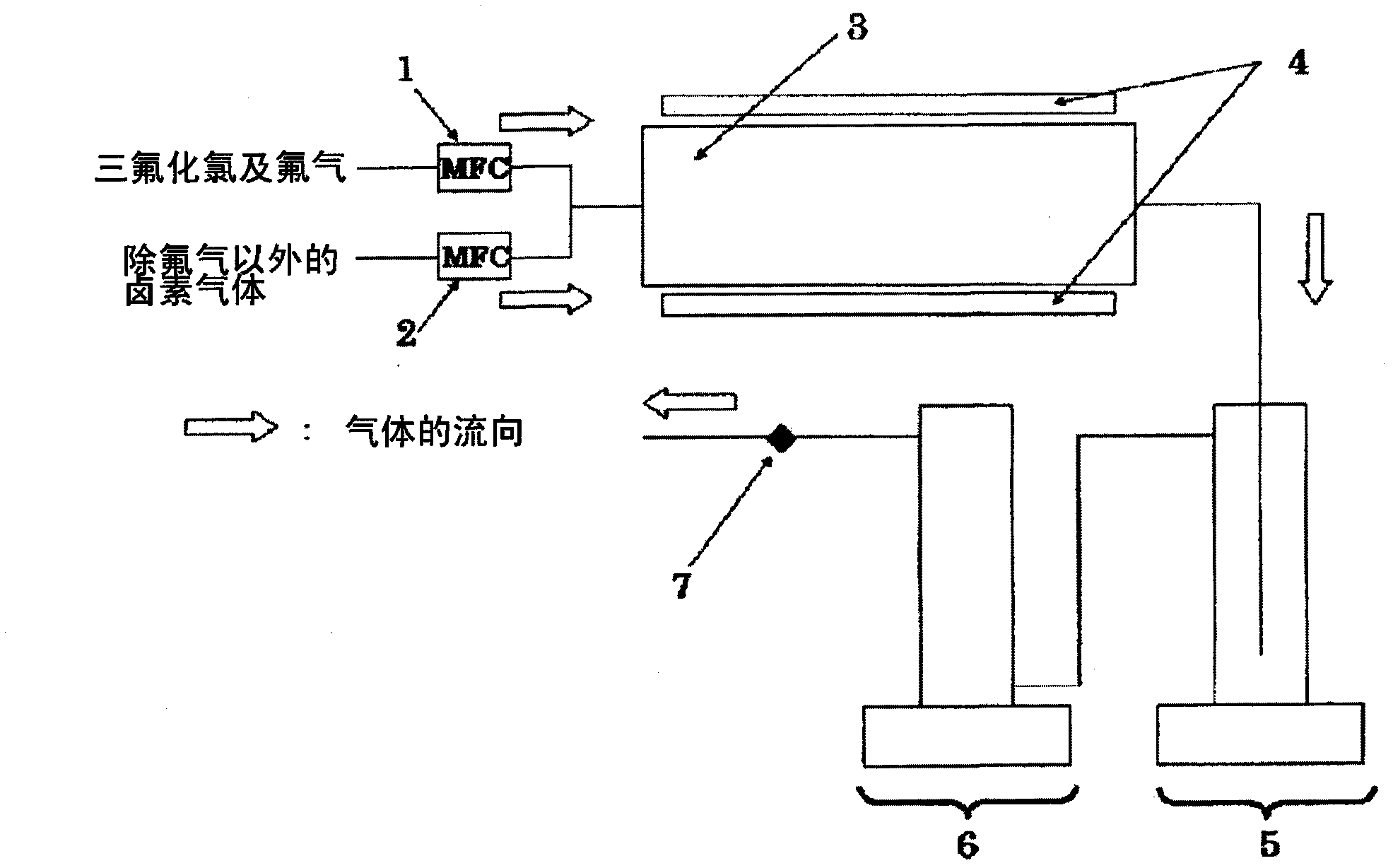
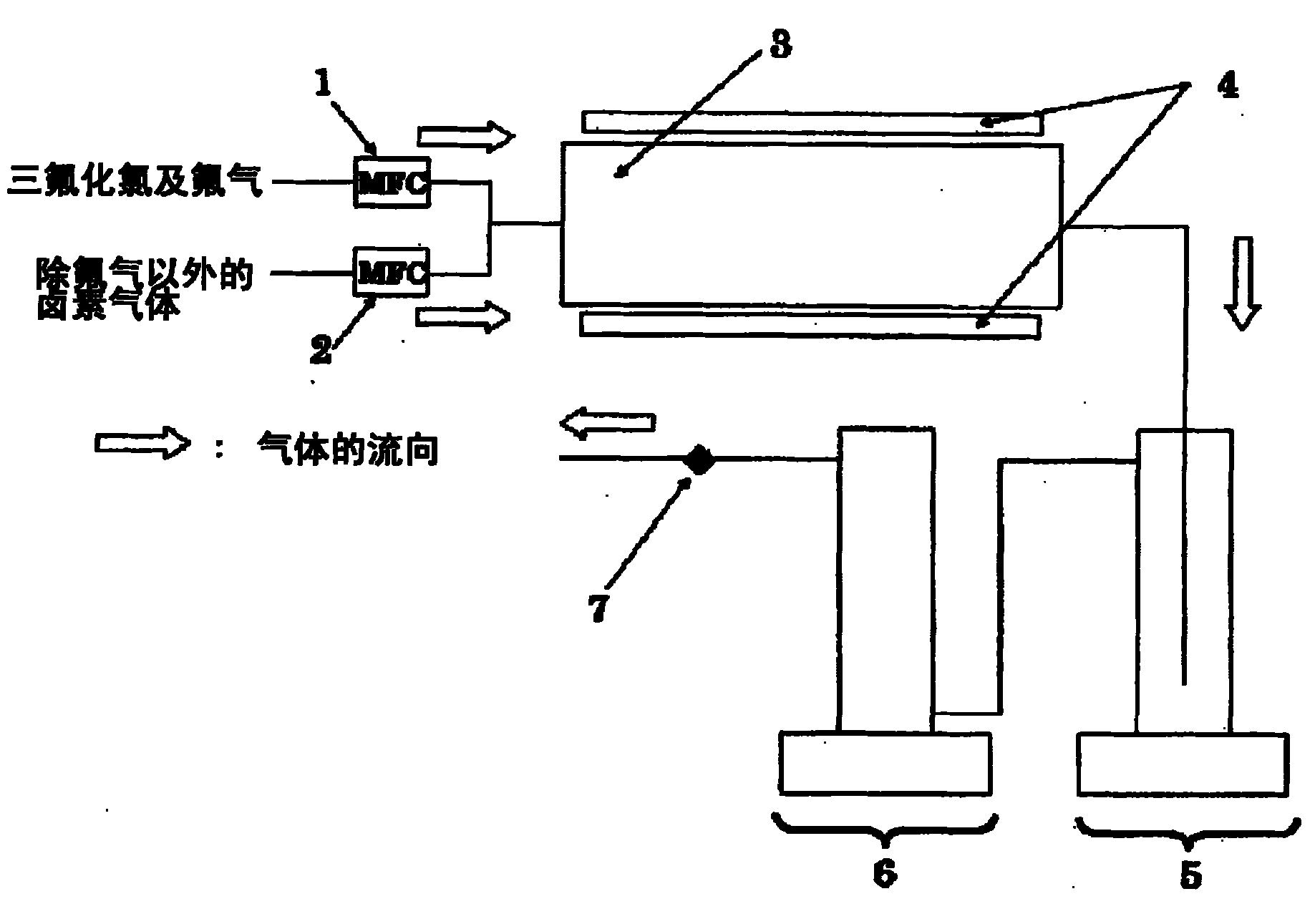
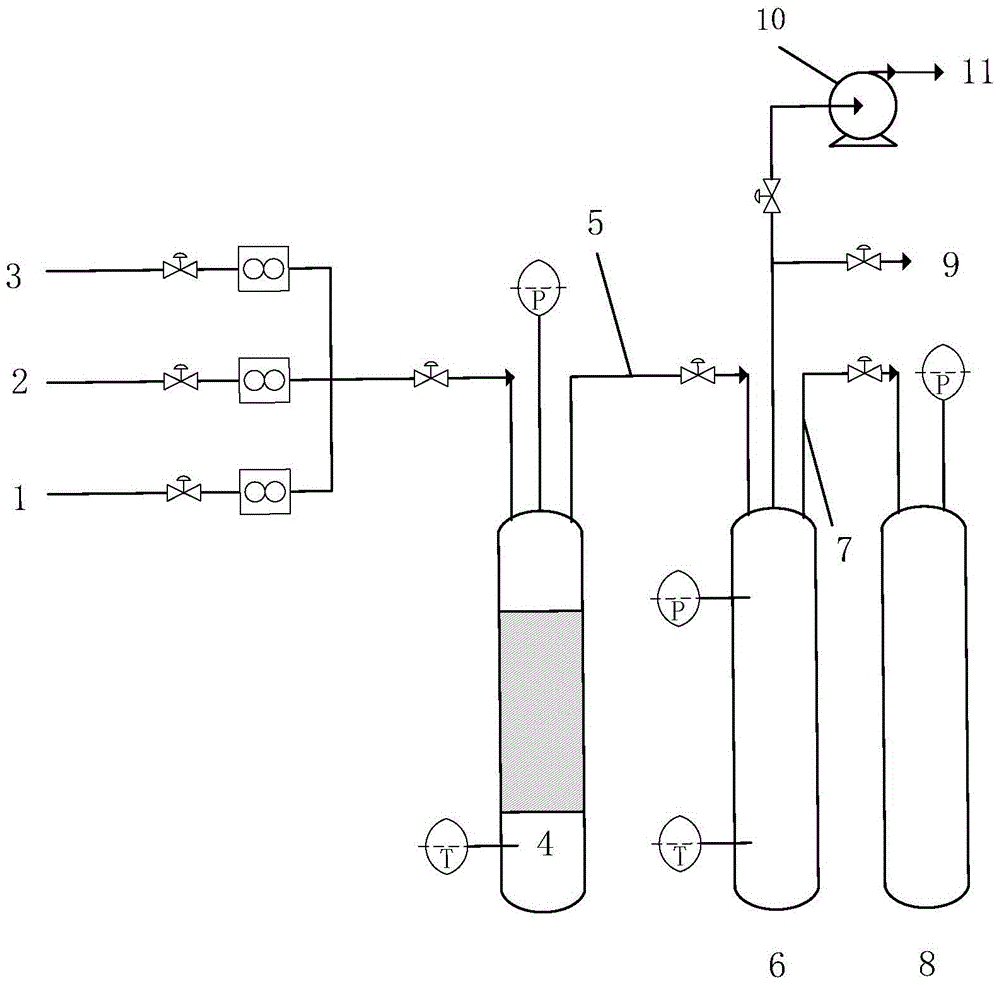
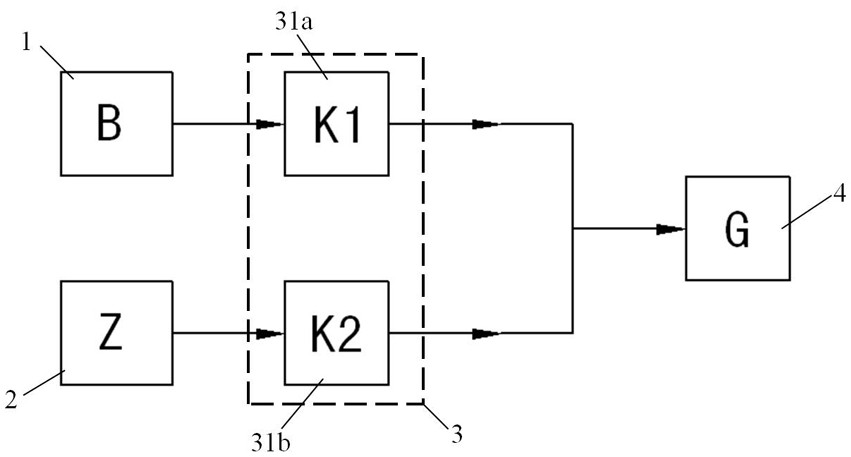
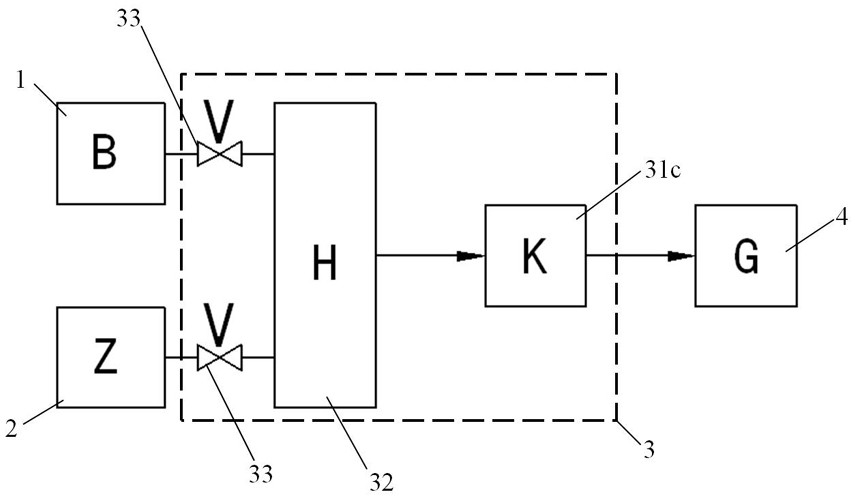
Bromine trifluoride and load gas batching method and device
PendingCN113262714ASimple methodEasy to installTransportation and packagingHollow article cleaningProcess engineeringBromine trifluoride
The invention discloses a bromine trifluoride and load gas batching method and device, and the device comprises a bromine trifluoride feeding container and a load gas feeding container which are communicated with a radioactive device through a pipeline and a regulation and control mechanism; the regulation and control mechanism can be a combination of a bromine trifluoride flow metering control device and a load gas flow metering controller device; and the device can also be a combination of a mixed gas flow metering controller device, a mixed batching container and a valve. According to different adjusting mechanisms, the batching method comprises the following two modes: respectively controlling and metering the flow rates of the two material flows, then mixing and supplying the material flows into radioactive equipment, and firstly mixing the two gas flows in proportion, then metering the flow rate of the mixed gas and supplying the mixed gas into the radioactive equipment. The device can be used for burdening of bromine trifluoride and load gas flow and proportion in a large range, and the burdening gas flow and proportion can be controlled accurately; and the method and the device are simple, convenient and high in reliability.
Owner:RES INST OF PHYSICAL & CHEM ENG OF NUCLEAR IND
Method for preparing boron-10 acid from boron trifluoride-10
InactiveCN104310420BHigh yieldHigh purityBoron oxyacidsBoron halogen compoundsAlcoholLithium hydroxide
Owner:TIANJIN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com