Method for preparing bromine trifluoride
A technology for bromine trifluoride and crude bromine trifluoride, which is applied in the direction of interhalogen compounds, can solve the problems of high impurity content of bromine trifluoride, serious environmental pollution, and high equipment requirements, and achieve low environmental pollution, simple equipment, The effect of equipment operation safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
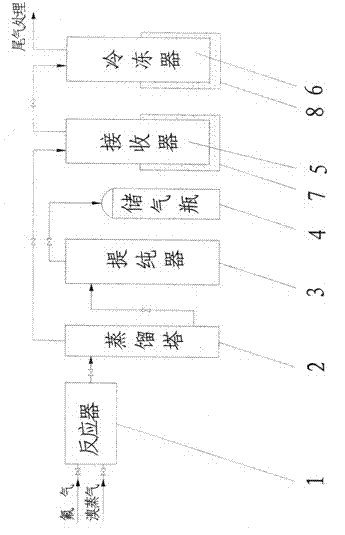
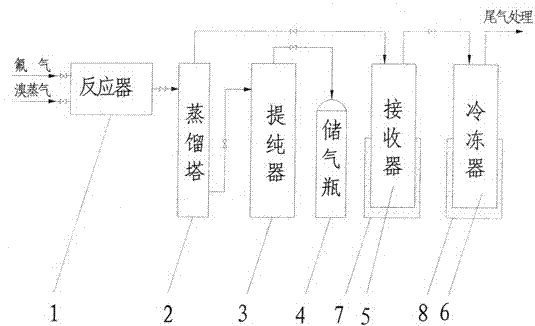
[0052] Clean and dry the reactor, preheat the reactor to 100°C and evacuate to -0.095Mpa after passing the leak test for the seal of the reactor. Slowly open the valve in front of the reactor, feed fluorine gas into the reactor, and close the valve in front of the reactor after the pressure of the reactor reaches normal pressure. Open the inlet valve of the bromine raw material, feed bromine vapor and fluorine gas to carry out the synthesis reaction, and generate crude bromine trifluoride containing impurities. The reactor temperature was controlled at 120°C. After the reaction, the reactor pressure did not drop any more.
[0053] The bromine trifluoride liquid coming out of the reactor enters the distillation tower, and the distillation tower is heated to a temperature of 70°C. Open the valve in front of the receiver, and light impurities such as bromine pentafluoride and bromine simple substance enter the receiver from the top of the distillation tower for liquefaction. Th...
Embodiment 2
[0056] Clean and dry the reactor, preheat the reactor to 110°C and evacuate to -0.095Mpa after passing the leak test for the seal of the reactor. Slowly open the valve in front of the reactor, feed fluorine gas into the reactor, and close the valve in front of the reactor after the pressure of the reactor reaches normal pressure. Open the inlet valve of the bromine raw material, feed bromine vapor and fluorine gas to carry out the synthesis reaction, and generate crude bromine trifluoride containing impurities. The reactor temperature was controlled at 140°C. After the reaction, the reactor pressure did not drop any more.
[0057] The bromine trifluoride liquid coming out of the reactor enters the distillation tower, and the distillation tower is heated to a temperature of 80°C. Open the valve in front of the receiver, and light impurities such as bromine pentafluoride and bromine simple substance enter the receiver from the top of the distillation tower for liquefaction. Th...
Embodiment 3
[0060]Clean and dry the reactor, preheat the reactor to 120°C and evacuate to -0.095Mpa after passing the leak test for the seal of the reactor. Slowly open the valve in front of the reactor, feed fluorine gas into the reactor, and close the valve in front of the reactor after the pressure of the reactor reaches normal pressure. Open the inlet valve of the bromine raw material, feed bromine vapor and fluorine gas to carry out the synthesis reaction, and generate crude bromine trifluoride containing impurities. The reactor temperature was controlled at 160°C. After the reaction, the reactor pressure did not drop any more.
[0061] The bromine trifluoride liquid coming out of the reactor enters the distillation tower, and the distillation tower is heated to a temperature of 90°C. Open the valve in front of the receiver, and light impurities such as bromine pentafluoride and bromine simple substance enter the receiver from the top of the distillation tower for liquefaction. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com