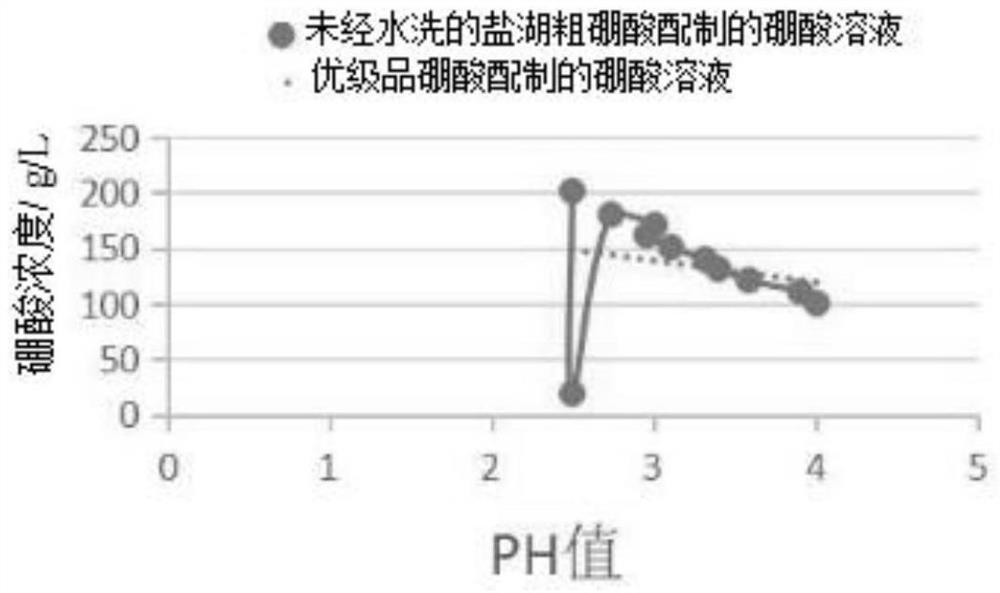
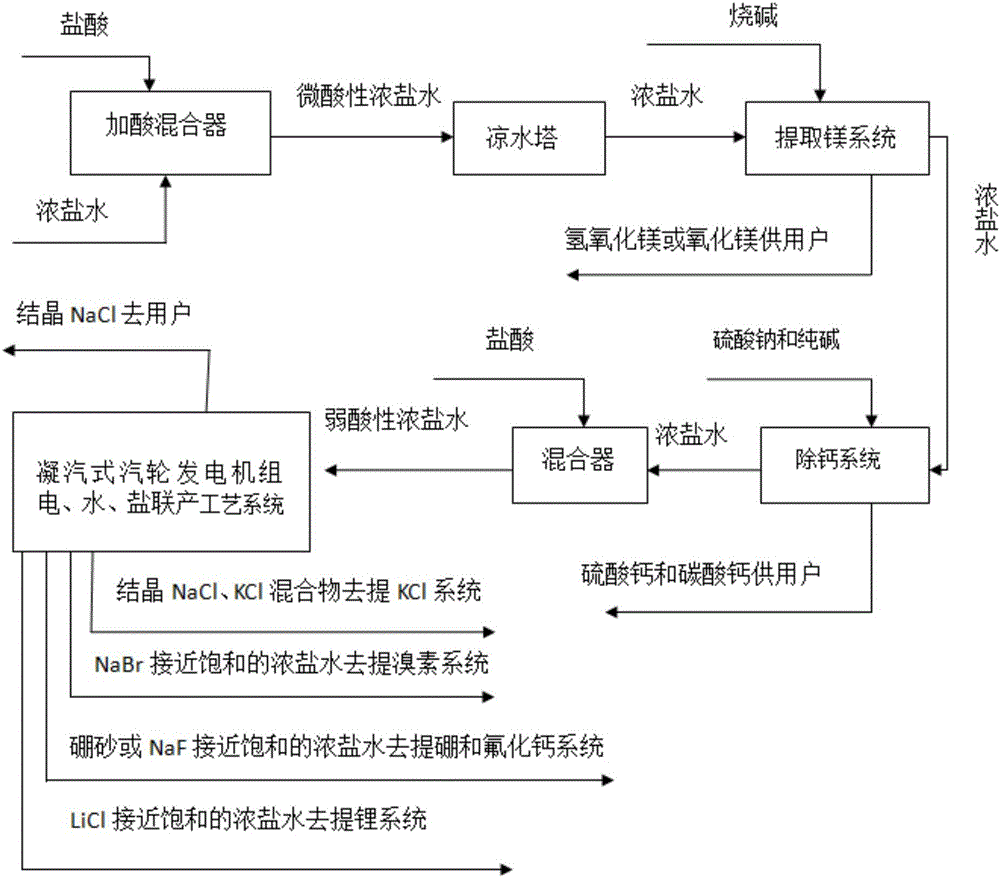
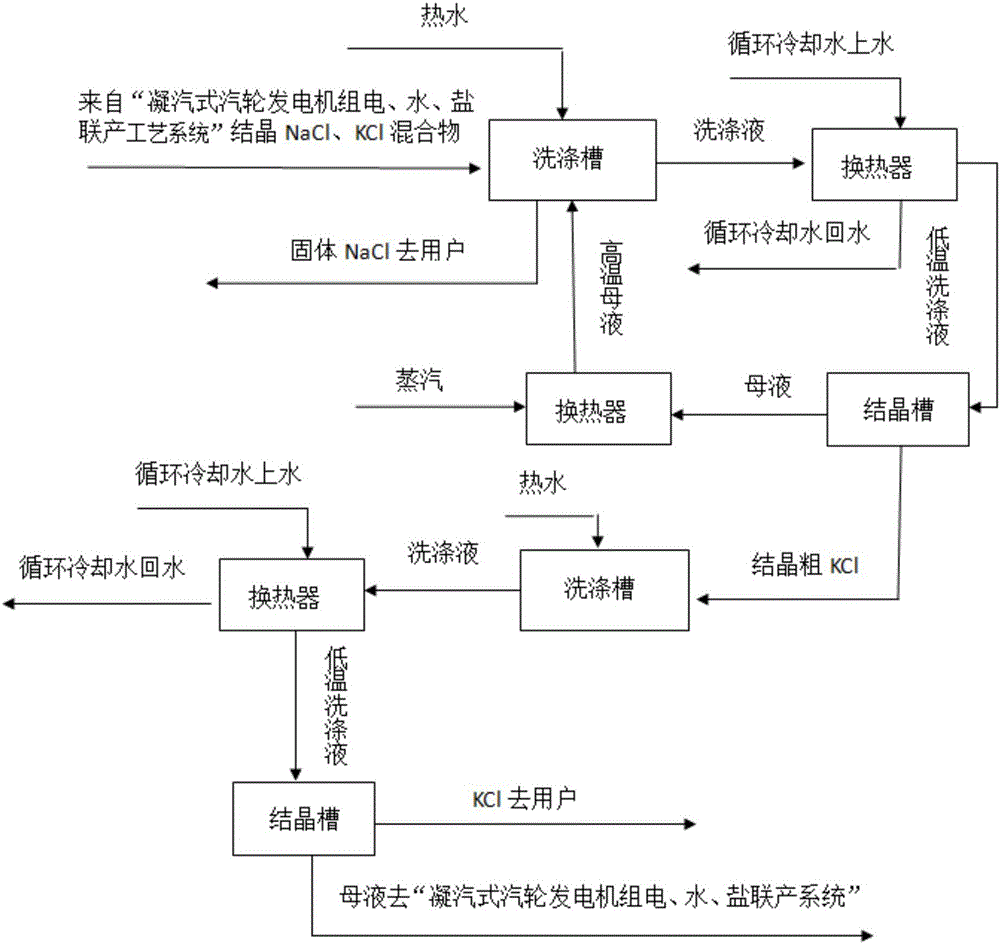
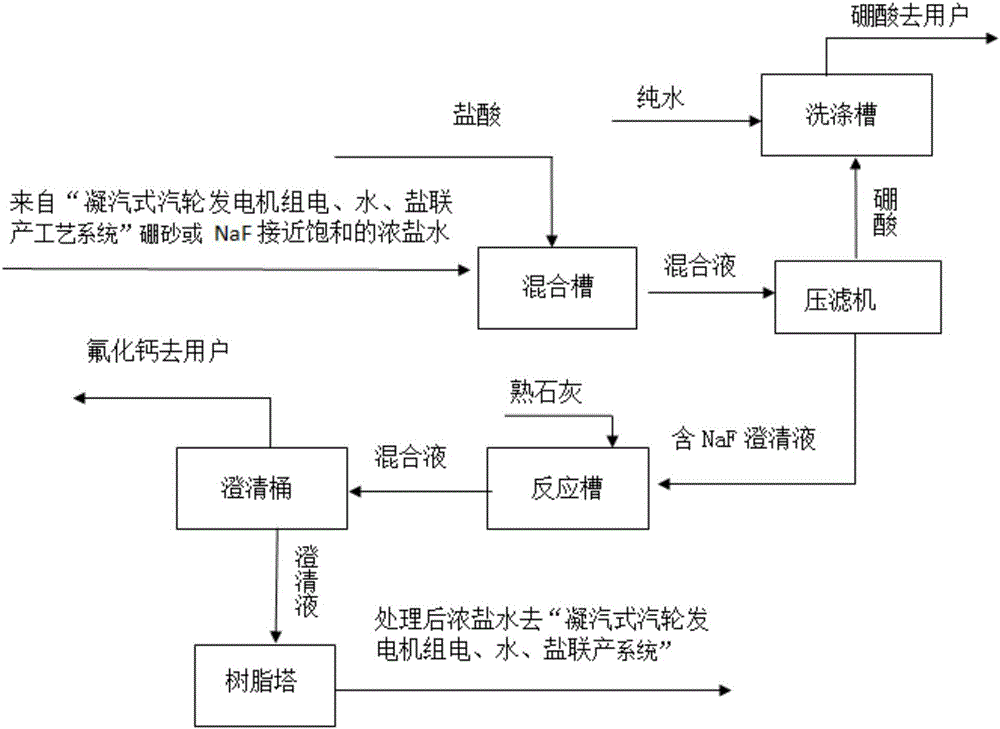
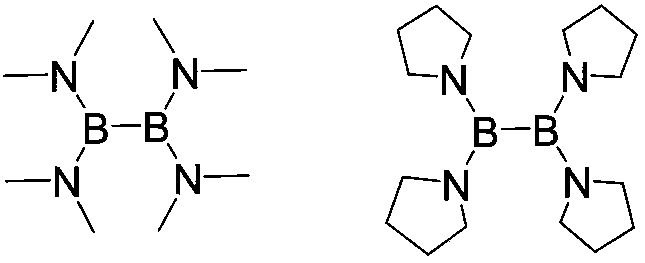
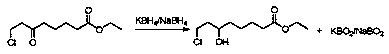Patents
Literature
58results about "Boron oxyacids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
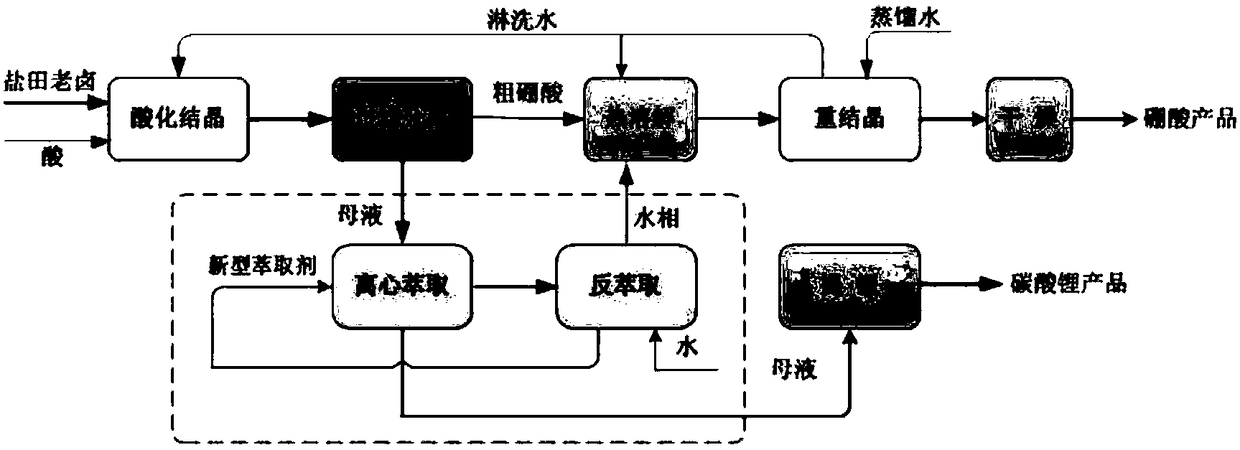
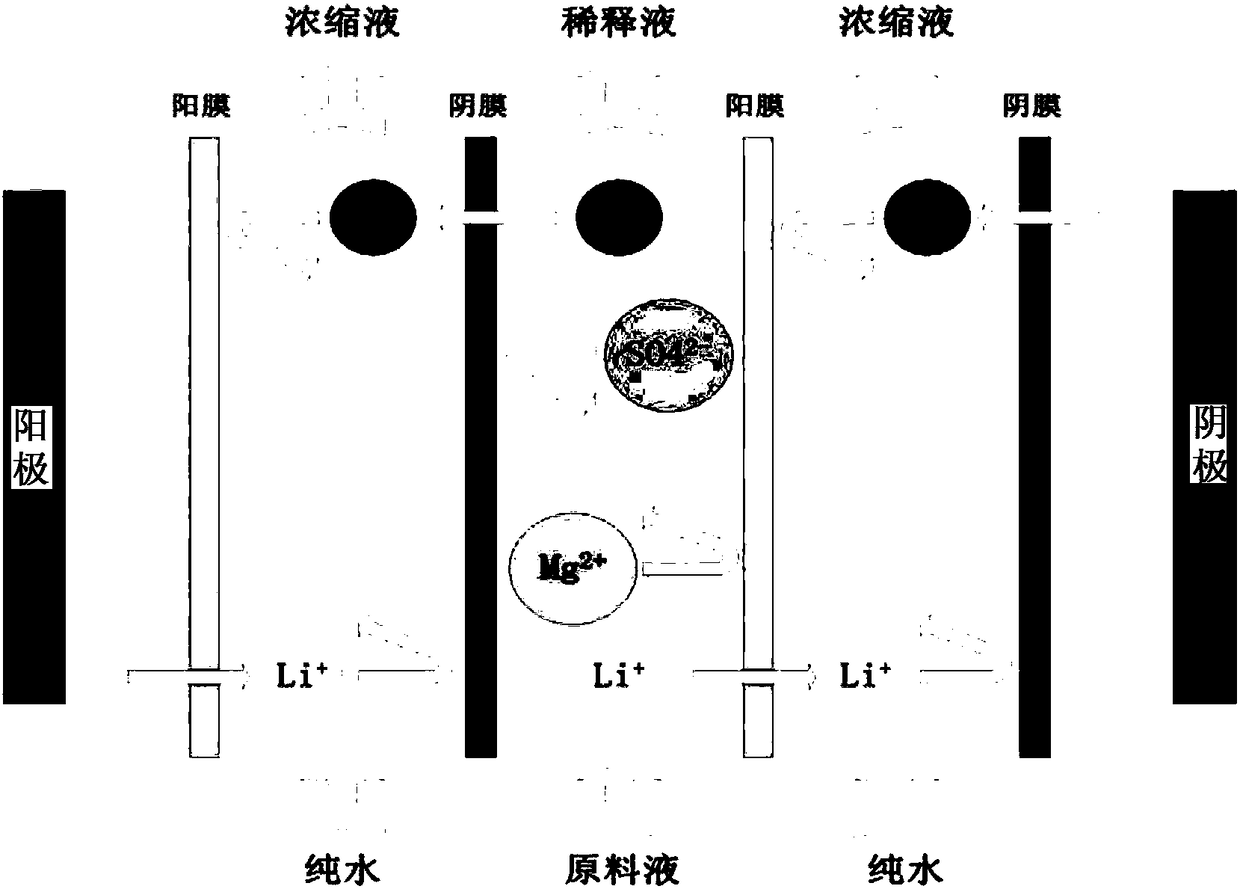
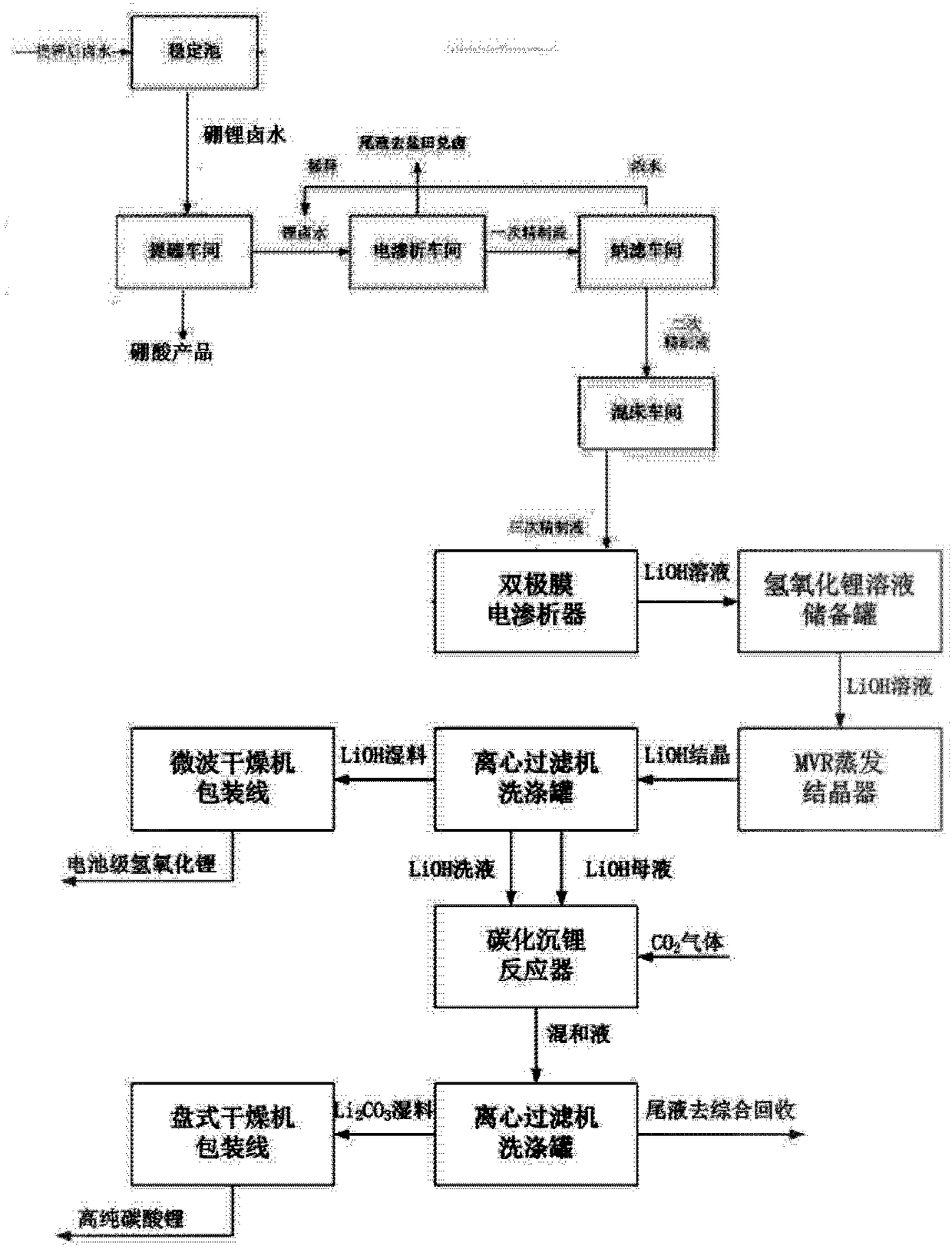
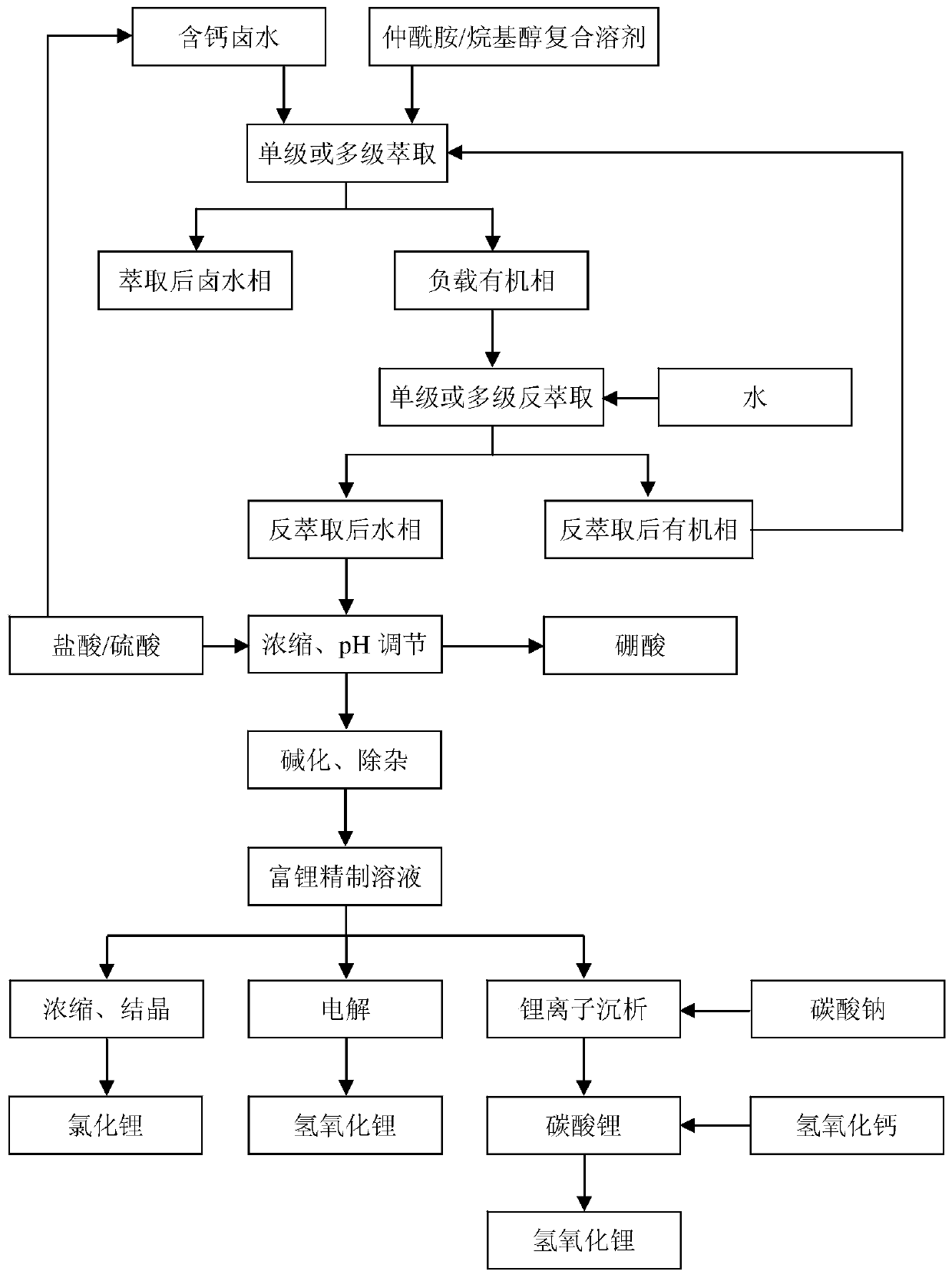
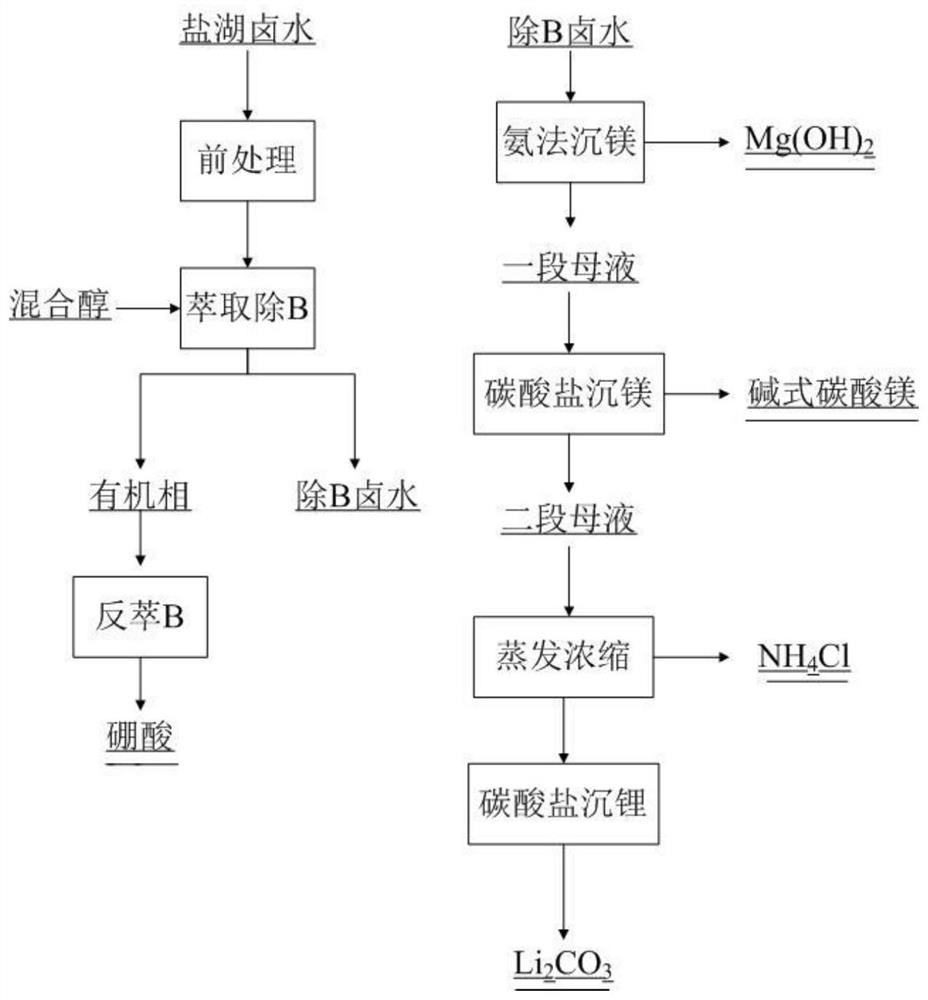
Method for directly preparing lithium hydroxide and lithium carbonate from salt lake brine with high magnesium-to-lithium ratio
ActiveCN108341420AEasy to operateEfficient extractionBoron oxyacidsLithium oxides/hydroxidesPotassiumEvaporation
The invention discloses a method for directly preparing lithium hydroxide and lithium carbonate from salt lake brine with a high magnesium-to-lithium ratio. The method comprises the following steps: 1, further stabilizing brine obtained after potassium extraction of a salt pan in a stabilization pond to form boron and lithium brine with low potassium and sodium content; 2, carrying out boron extraction treatment on the boron and lithium brine to form a boric acid product and lithium brine; 3, 4 and 5, allowing the lithium brine to go through three times of refining to obtain a thirdly refinedsolution; 6, allowing the thirdly refined solution to go through a bipolar membrane electrodialyzer to form a lithium hydroxide solution; 7, allowing the lithium hydroxide solution to go through an evaporation crystallizer to obtain a lithium hydroxide monohydrate solid and evaporation mother liquor; 8, washing the lithium hydroxide monohydrate solid for recrystallization to form battery grade lithium hydroxide and a washing solution; and 9, allowing the evaporation mother liquor and the washing solution to go through a gas-liquid reactor to react with carbon dioxide gas to form the lithium carbonate. The method has the advantages of good maneuverability, and great increase of the recovery rate of lithium ions.
Owner:马培华
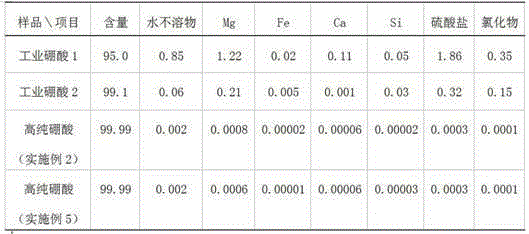
Preparation method of high-purity boric acid
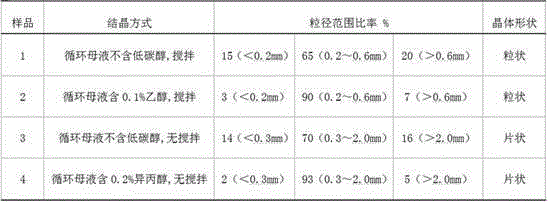
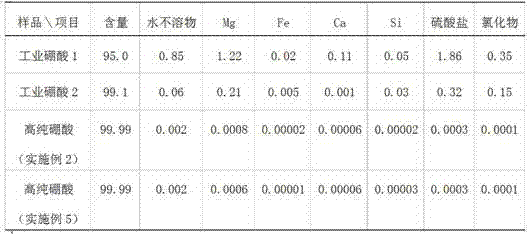
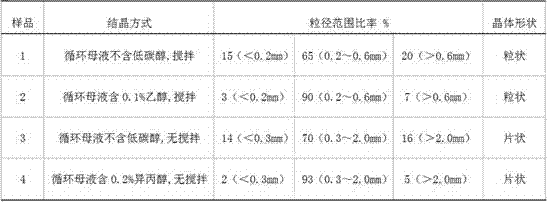
ActiveCN105347353ANo pollution in the processSimple processBoron oxyacidsLamellar crystalsPhysical chemistry
The invention provides a preparation method of high-purity boric acid. The preparation method comprises the following steps: putting industrial boric acid into a mixed solution of circulating mother liquor and trace inorganic acid, stirring, and heating for dissolution; performing thermal reaction for 1-2 hours in a temperature range of 80-95 DEG C; cooling to obtain wet boric acid crystals; respectively washing the crystals by virtue of mother liquor alternately purified by a cation exchange resin column and an anion exchange resin column; and drying to prepare high-purity boric acid. Compared with the prior art, the technical scheme provided by the invention has the technical advantages of being simple in process flow, having no environmental pollution, being high in crystal purity and low in manufacturing cost, selecting between granular or lamellar crystals, and the like; moreover, the crystal particle size distribution can be greatly improved.
Owner:DANDONG CHEM REAGENT FACTORY
Method for preparing high-purity secondary boric acid
The invention relates to a method for preparing high-purity secondary boric acid. The method comprises the following steps: preparing rough boric acid into an unsaturated solution, heating to dissolve boric acid, filtering the hot dissolved boric acid by using quartz sand, cooling, injecting the filtrate into a treated strong-acid strong-alkali mixing bed ion exchange column, controlling the flowing speed, heating and concentrating a solution (which is subjected to ion exchange) at a low temperature, filtering by using a microfiltration membrane, putting into a sealed plastic cup, cooling in a low-temperature constant-temperature tank to separate out boric acid crystals, performing centrifugal separation, carrying out suction filtering in vacuum, washing, and drying in vacuum, thus obtaining secondary boric acid. By adopting the method, the price of raw materials is low, a small amount of byproducts are generated, the energy consumption is low, and high-purity secondary boric acid is easy to prepare.
Owner:重庆市盛馨种业有限公司
Preparation method of high-purity boric acid
ActiveCN105347353BNo pollution in the processSimple processBoron oxyacidsLamellar crystalsDissolution
The invention provides a preparation method of high-purity boric acid. The preparation method comprises the following steps: putting industrial boric acid into a mixed solution of circulating mother liquor and trace inorganic acid, stirring, and heating for dissolution; performing thermal reaction for 1-2 hours in a temperature range of 80-95 DEG C; cooling to obtain wet boric acid crystals; respectively washing the crystals by virtue of mother liquor alternately purified by a cation exchange resin column and an anion exchange resin column; and drying to prepare high-purity boric acid. Compared with the prior art, the technical scheme provided by the invention has the technical advantages of being simple in process flow, having no environmental pollution, being high in crystal purity and low in manufacturing cost, selecting between granular or lamellar crystals, and the like; moreover, the crystal particle size distribution can be greatly improved.
Owner:DANDONG CHEM REAGENT FACTORY
Method for separating boron isotopes by using MOF-74(Zn) as simulated moving bed stationary phase
InactiveCN107413195AHigh separation factorLow costIsotope separationBoron oxyacidsSimulated moving bedMoving bed
The invention discloses a method for separating boron isotopes by using MOF-74(Zn) as a simulated moving bed stationary phase. The method comprises the following steps: firstly, filtering a boric acid aqueous solution and a hydrochloric acid aqueous solution, then removing impurities and degassing; secondly, a simulated moving bed comprises I to IV areas, wherein 3 to 7 chromatographic columns are arranged in each area; taking the MOF-74(Zn) as the simulated moving bed in stationary phase; setting the flow rates of the I to IV areas, port switching time and operating temperature; continuously adding the boric acid aqueous solution into the chromatographic columns of the simulated moving bed, and eluting with the hydrochloric acid aqueous solution; after the simulated moving bed reaches the equilibrium state, collecting the boric acid aqueous solution enriched with isotope 11B at an extraction opening of the simulated moving bed and collecting the boric acid aqueous solution enriched with isotope 10B at the extraction opening of the simulated moving bed. The method disclosed by the invention is a method for continuously separating the boron isotopes and has the advantages of simple operation and relatively-high separation efficiency; in addition, the cost of concentrated isotope 10B can be effectively reduced and relatively-high boron isotope separation factors are obtained.
Owner:TIANJIN UNIV
Boracic acid recovery and reutilization method in lactulose preparation
InactiveCN106589006ASimple operation processEasy to industrializePhysical/chemical process catalystsSugar derivativesIsomerizationBoron containing
The invention discloses a boracic acid recovery and reutilization method in the lactulose preparation process. The method mainly comprises the steps of (1) performing nanofiltration on an isomerized lactulose solution for removing salts and boracic acid; (2) concentrating, crystallizing and drying nanofiltration permeate liquid to obtain boracic acid crystals; and (3) adding the boracic acid crystals back into the lactulose solution as a catalyst; and performing an isomerization reaction. The method provided by the invention has the advantages that the removal rate of the boracic acid in the isomerized solution through nanofiltration reaches 98 percent or higher; after the crystallization treatment, the boracic acid can be recovered and reused again; the recovery and reutilization rate is higher than 85 percent; the operation process is simple; the industrialization can be easily realized; meanwhile, the production cost is greatly reduced; the step of treating a large amount of boron-containing water is omitted; the sewage discharge quantity is reduced; and a better environment-friendly effect is achieved.
Owner:BAOLINGBAO BIOLOGY
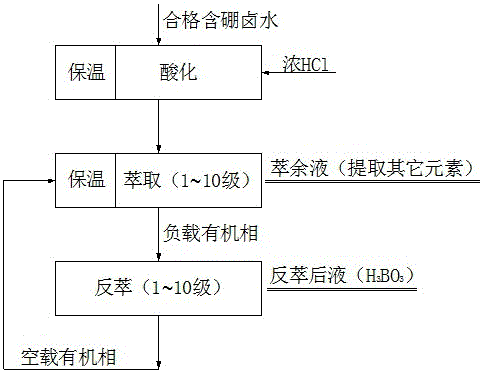
Method for extracting boron with combination of solvent extraction-ion exchange adsorption
ActiveCN104445242ARealize development and utilizationReduce dosageBoron oxyacidsMagnesium saltIon exchange
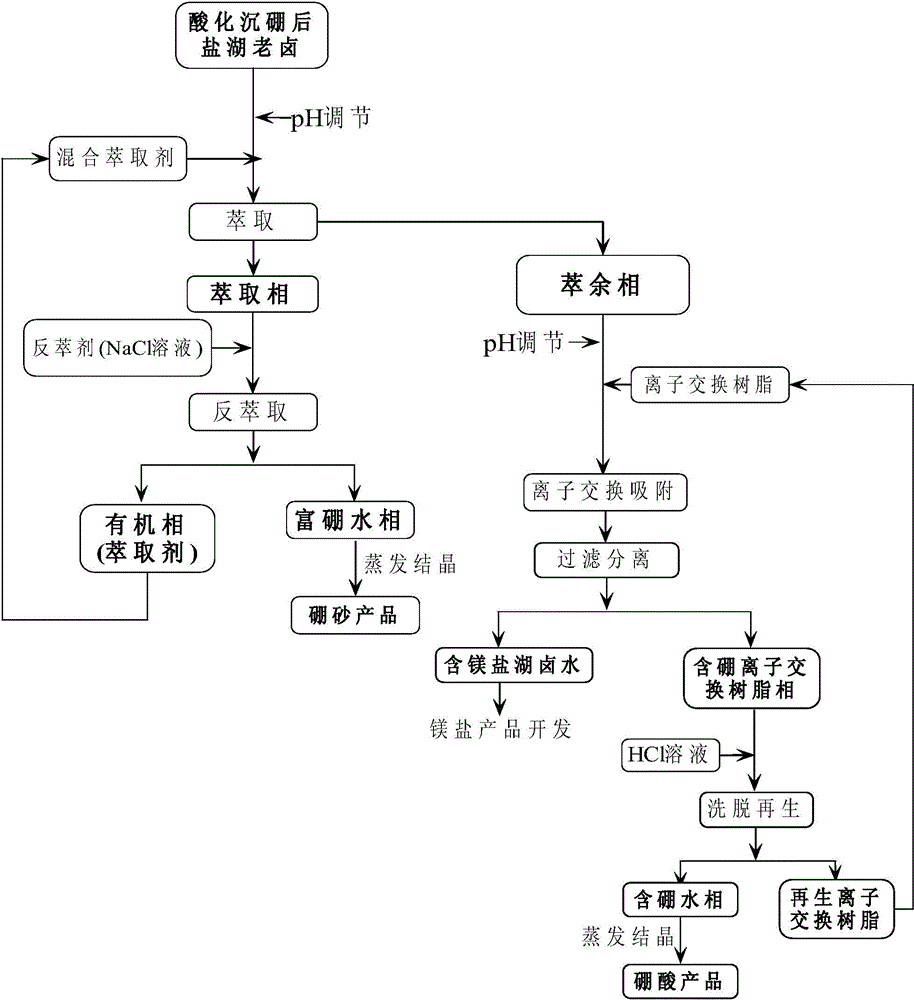
The invention relates to a method for extracting boron with the combination of solvent extraction-ion exchange adsorption. According to the method, boric acid of relatively low content is extracted from old brine of a magnesium-containing salt lake after acidification boron precipitation; the energy consumption can be effectively reduced as an extraction procedure with relatively small grade numbers is adopted, and the use amount of an extraction agent is reduced; after extraction, an ion exchange adsorption procedure is performed, and the trace boron element is removed by using an ion exchange adsorption method with high selectivity, so that the boron extraction grade number is effectively reduced, and the energy consumption is reduced; as a novel mixed extracting agent system is adopted, the single-grade extraction rate is high, and the solution loss is small. The method can be implemented under the condition of low energy consumption, the boron of low content in old brine after acidification boron precipitation of the salt lake can be effectively extracted, the extract is a pure boron-containing solution, a high-quality boron product can be prepared, the boron resource can be sufficiently utilized, the quality of a later magnesium salt product is greatly improved, comprehensive development and utilization of the old brine after acidification boron precipitation of the salt lake are achieved, and the economic benefits are remarkably increased.
Owner:TIANJIN UNIV
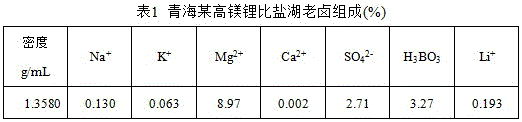
Extraction system for extracting lithium and boron by separating calcium from calcium-containing brine by secondary amid/alkyl alcohol composite solvent, extraction method and application of extraction method
ActiveCN110669947ASimple structureConvenient sourceProcess efficiency improvementBoron oxyacidsLithium chlorideAlcohol
The invention discloses an extraction system for extracting lithium and boron by separating calcium from calcium-containing brine by a secondary amid / alkyl alcohol composite solvent, an extraction method and application of the extraction method. In the extraction system, secondary amid and alkyl alcohol serve as extracting agents for extracting lithium and boron correspondingly and consist of single compound or a mixture of more than two compounds; the total number of carbon atoms in the molecules is 12 to 18 and 8 to 20 correspondingly, and the freezing point of the extraction system is lessthan 0 DEG C. Single-stage or multi-stage countercurrent extraction is conducted under the conditions that the volume ratio of an organic phase to a brine phase is (1-10):1, the density of the brine is 1.30 to 1.56 g / cm<3>, the pH value of the brine is 0 to 7 and the temperature is 0 to 50 DEG C, an aqueous phase with low calcium-lithium ratio is obtained through back extraction, and lithium chloride, lithium carbonate, lithium hydroxide and boric acid are obtained respectively through concentration, impurity removal and preparation. The secondary amide is simple in molecular structure, and the lithium and the boron can be extracted simultaneously by the composite solvent improved by the alkyl alcohol; multi-stage extraction rate is high, back extraction is conducted by using water and acid and alkali consumption is greatly reduced; and the extraction separation flow is shortened, and the extraction system has small solution loss and is suitable for development of oilfield brine.
Owner:XIANGTAN UNIV
Application of box-type extraction tank in extracting boron in salt lake brine
The invention provides an application of a box-type extraction tank in extracting boron in salt lake brine. The application includes two mainly sections of extraction and reverse extraction. The extraction tank is low in price, convenient in operation and easy to maintain, large-scale production is easy to control, simpleness in entire process and short circulation are achieved, boric acid products more than 99.9% in purity can be obtained via simple evaporating, cooling and crystallizing of obtained reverse extraction liquid, and promotion and application in Qinghai Chaidamu areas are facilitated.
Owner:青海柴达木兴华锂盐有限公司
Method for preparing boron fertilizer
InactiveUS20120088666A1Improve the level ofFast dissolutionBiocideAmmonium nitratesDissolutionCooling down
A method for preparing a boron fertilizer, including: (1) heating boric acid to a temperature of 180-200° C., maintaining the temperature for 20-30 min for dehydration of the boric acid to yield pyroboric acid; and (2) cooling down the pyroboric acid to a temperature of 40-60° C., crushing, and screening to yield a powdered, weakly acidic, high-content boron fertilizer. The method is energy-saving, environmentally friendly, and low in cost. The resulting boron fertilizer is weakly acidic, fast in dissolution rate, and has excellent in compounding performance
Owner:UKHAN FISHERI EHGRIKALCHERAL SAJENS EHND TECH KO
Salicylic acid functionalized boron chelating adsorbent preparation method, product prepared therethrough, and application of product
InactiveCN107159165AEfficient extractionSimple preparation processOther chemical processesBoron oxyacidsSorbentSalicylic acid
The invention discloses a salicylic acid functionalized boron chelating adsorbent preparation method, a product prepared therethrough, and an application of the product. The preparation method comprises the following steps: mixing a functional component, phenol, formaldehyde, concentrated hydrochloric acid and a pore forming agent in proportion to obtain a water phase, carrying out a pre-polycondensation reaction on the water phase, transferring the obtained pre-polycondensation reaction product to an oil phase, carrying out reversed phase suspension polymerization to obtain a primary product, carrying out Soxhlet extraction on the primary product to obtain the purified salicylic acid functionalized boron chelating adsorbent, wherein the functional component is at least one of salicylic acid and derivatives thereof. The salicylic acid functionalized boron chelating adsorbent prepared in the invention has the advantages of high specific selectivity, short adsorption balancing time and high adsorption efficiency, and is applied to the extraction of boron from water. Aromatic salicylic acid and the derivatives thereof are adopted as the boron adsorption functional component, and the target product is obtained through the reversed phase suspension polymerization and the one-step polycondensation, so the preparation method has the advantages of simplicity, mild conditions, and realization of high-efficiency extraction of boric acid from water.
Owner:ZHEJIANG UNIV
Lithium iron phosphate-boric acid co-coated lithium nickel cobalt aluminate positive electrode material and preparation method thereof
InactiveCN111653755ATotal base reductionEasy to processSecondary cellsPositive electrodesElectrolytic agentLithium iron phosphate
The invention discloses a lithium iron phosphate-boric acid co-coated lithium nickel cobalt aluminate positive electrode material and a preparation method thereof. The preparation method comprises thefollowing steps: weighing a certain mass of a lithium nickel cobalt aluminate precursor, mixing lithium, sintering to obtain a lithium nickel cobalt aluminate primary sintered material, carrying outstirring and water washing on the lithium nickel cobalt aluminate primary sintered material, and carrying out vacuum drying to obtain a lithium nickel cobalt aluminate positive electrode material matrix; and mixing the matrix positive electrode material with a coating material lithium iron phosphate-boric acid, and carrying out high-temperature sintering to obtain the lithium iron phosphate-boricacid co-coated lithium nickel cobalt aluminate positive electrode material. The co-coated lithium nickel cobalt aluminate positive electrode material provided by the invention has good conductivity and a uniform coating layer, can prevent direct reaction between a positive electrode material matrix and an electrolytic solution, and effectively improves the cycle performance and safety performanceof the material. The method is simple in process, excellent in product performance, low in cost and suitable for large-scale industrial production.
Owner:SHAANXI COAL & CHEM TECH INST
Boric acid composite extractant and method for recovering boric acid, magnesium and lithium from salt lake old brine
InactiveCN112390266AAchieve high recovery extractionHigh single-stage extraction rateMagnesium carbonatesLiquid solutions solvent extractionChemical industryIsooctyl alcohol
The invention belongs to the field of salt lake chemical industry, and particularly relates to a boric acid composite extractant, which comprises isooctyl alcohol, isoamyl alcohol and 2-ethyl-1, 3-hexanediol. The invention also provides a method for extracting boric acid from salt lake old brine by adopting the boric acid composite extractant, and a method for extracting boron, magnesium and lithium from salt lake old brine through gradient separation. The invention innovatively finds that isooctyl alcohol, isoamyl alcohol and 2-ethyl-1, 3-hexanediol have unexpected synergism in the aspect ofboric acid extraction, and the single-stage extraction rate of boric acid can be effectively improved.
Owner:CENT SOUTH UNIV
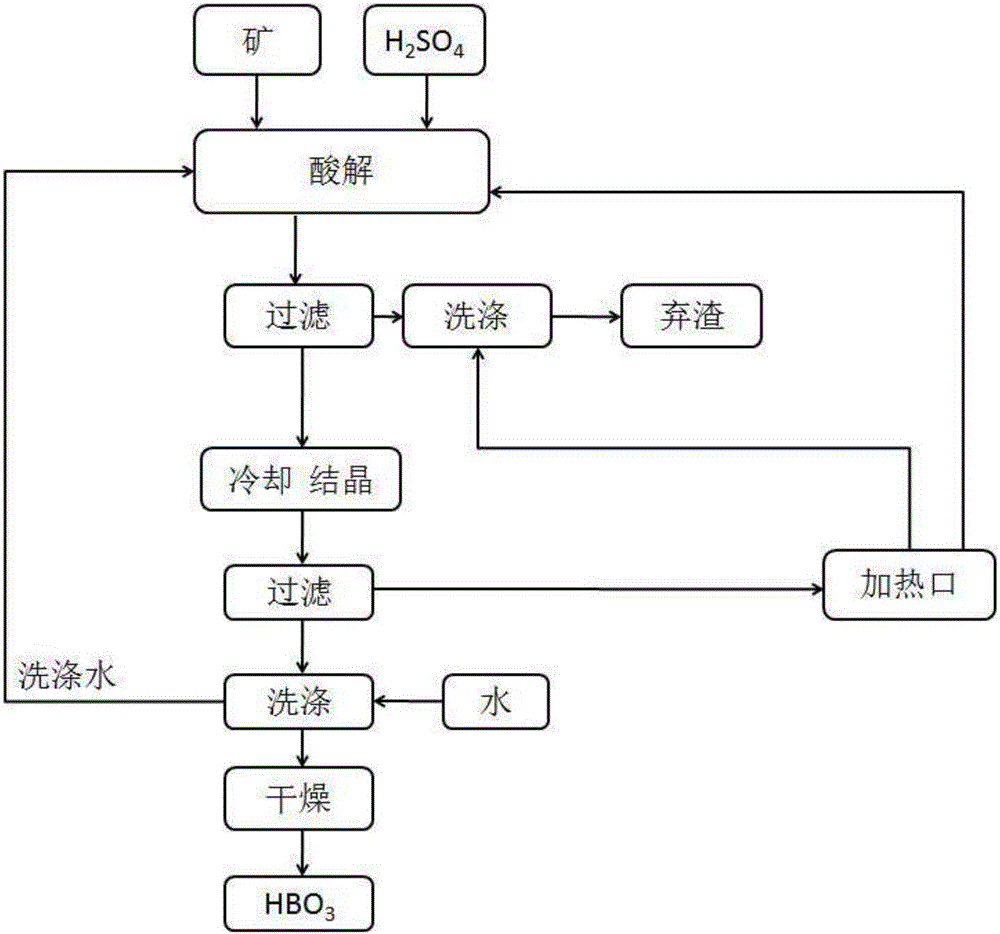
Method for preparing boric acid from ulexite
The invention provides a method for preparing boric acid from ulexite. The technical scheme includes that the method includes the technological process of acidolysis, crystallization, washing and drying, and production efficiency is improved by optimization of reaction conditions and process operation. The method includes the steps of 1), enabling concentrated sulfuric acid and ulexite powder to react as sufficiently as possible; 2), performing solid-liquid separation to remove CaSO4 2H2O, CaSO4 MgSO4 composite salt and sediment carried in ore; 3), cooling for crystallizing to separate out a boric acid coarse product, observing pH value change of the boric acid coarse product at different temperatures in the process, and properly adjusting a pH value to better control granularity and whiteness of the product; 4), performing solid-liquid separation to collect the solid boric acid coarse product, using water to wash the coarse product, and returning washing liquid to the first process to increase yield. The method is simple and convenient to operate, high in product purity, needless of adjusting process conditions according to types and content of impurities and especially suitable for production on a large scale higher than ten thousand tons.
Owner:泰誉威(天津)新材料科技有限公司
Surface modification method of high-nickel positive electrode material and modified high-nickel positive electrode material
ActiveCN114229917AEffectively remove residual alkaliRemoval of residual alkaliTungsten oxides/hydroxidesCell electrodesMetallurgyLithium-ion battery
The invention discloses a surface modification method of a high-nickel positive electrode material and a modified high-nickel positive electrode material, and relates to the technical field of lithium ion batteries. The surface modification method of the high-nickel positive electrode material comprises the following steps: carrying out acid pickling on the high-nickel positive electrode material, and then mixing and sintering with a coating agent, wherein the coating agent contains elements W, Al and B. The high-nickel positive electrode material is firstly subjected to acid pickling to effectively remove residual alkali, then specific coating elements are used for sintering, W and B enter a grain boundary and are used for filling the grain boundary so as to reduce the specific surface area of the material, Al is used for improving the stability of a material interface, and the capacity and cycling stability of the material can be remarkably improved after sintering. Therefore, the purpose of remarkably reducing the residual alkali of the material on the premise of ensuring the capacity and the cycling stability of the material is achieved.
Owner:宜宾锂宝新材料有限公司 +1
Method for treating boron-containing wastewater in 6-hydroxyl-8-chloro ethyl caprylate preparation process
InactiveCN108083528ALess investmentSimple methodWater contaminantsMultistage water/sewage treatmentPotassium borohydrideOrganic layer
The invention relates to a method for treating boron-containing wastewater in a 6-hydroxyl-8-chloro ethyl caprylate preparation process. According to the method for treating boron-containing wastewater in a 6-oxo-8-chloro ethyl caprylate preparation process, tetrabutylammonium bromide is used as a catalyst, a 6-oxo-8-chloro ethyl caprylate dichloroethane solution and a potassium borohydride ammonia water solution are subjected to a mixed reaction, 6-hydroxyl-8-chloro ethyl caprylate is obtained from the organic layer after the reaction, and ammonia water is recovered by concentrating the layer; and the method for treating boron-containing wastewater comprises: 1, after recovering the ammonia water, adding a proper amount of water, and adding an inorganic acid in a dropwise manner to acidify until the pH value is 1-2; 2, precipitating boric acid solid, filtering, drying the solid, and recovering; 3, evaporating the filtered boric acid mother liquor to remove a proper amount of water, cooling to a temperature of less than 30 DEG C, precipitating a potassium salt solid, drying the solid, and recovering; and 4, applying the mother liquor obtained in the step 3.
Owner:JIANGSU TOHOPE PHARMA
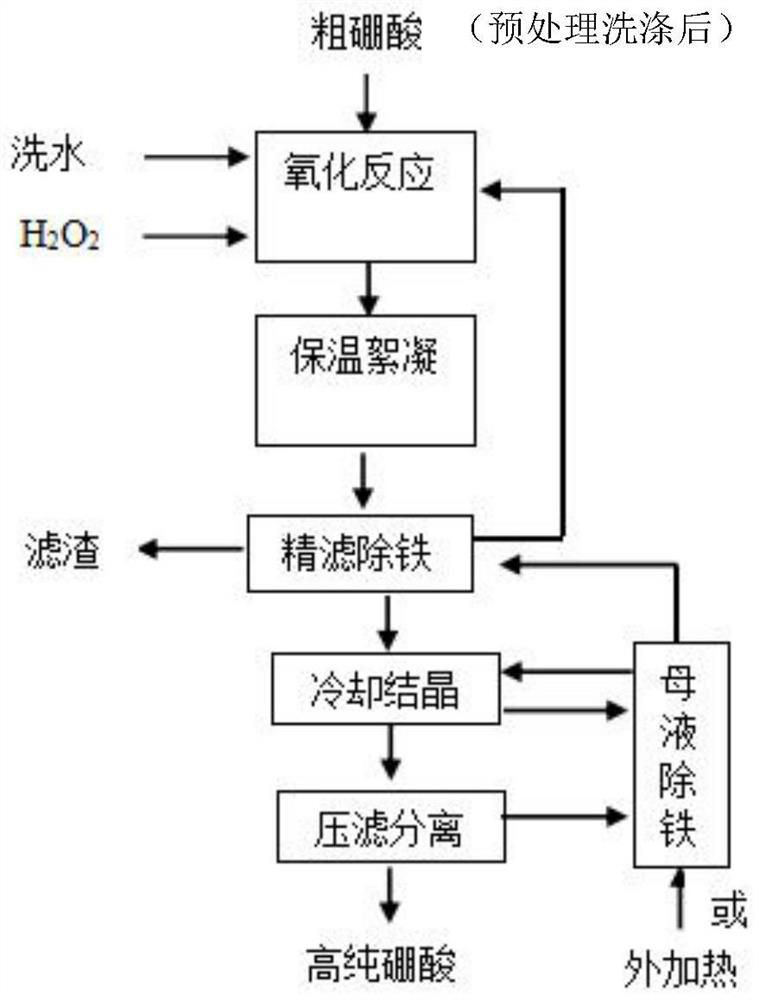
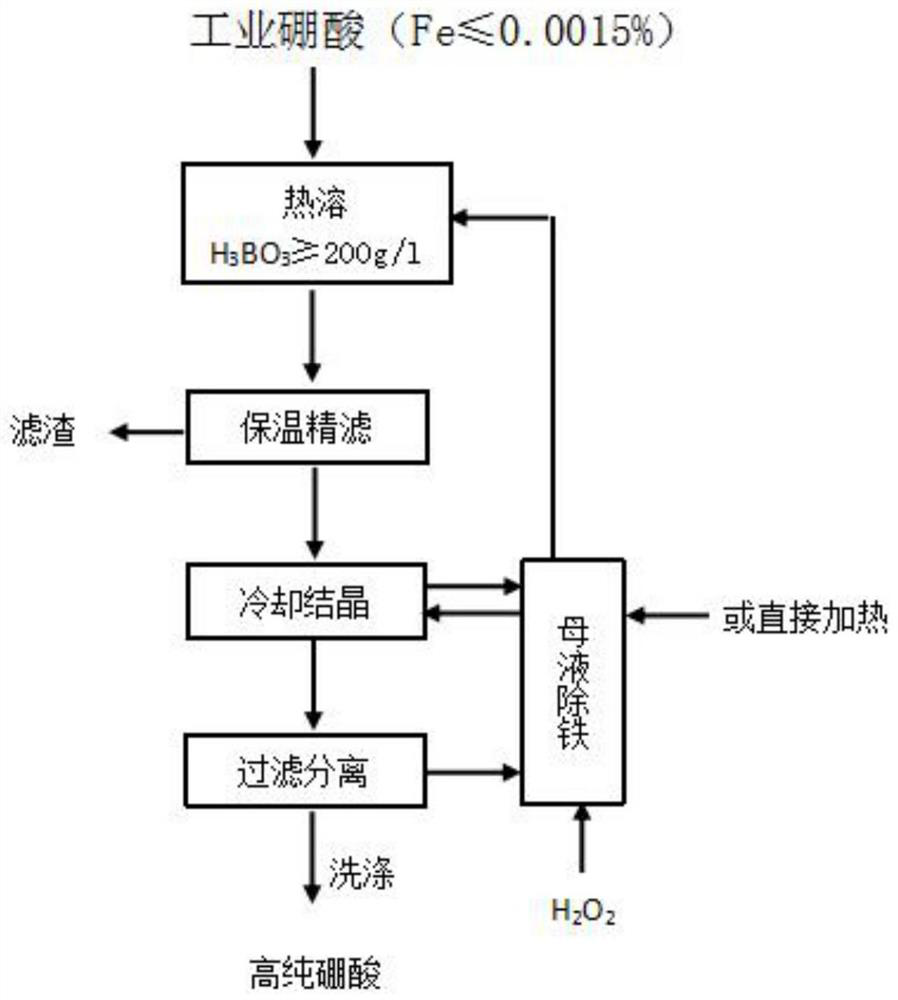
Iron removal method for preparing high-purity boric acid
ActiveCN113816393AEfficient removalTo achieve the purpose of iron removalEnergy inputBoron oxyacidsHigh concentrationFlocculation
The invention relates to the technical field of high-purity boric acid production, and provides an iron removal method for preparing high-purity boric acid. According to different iron contents in the crude boric acid (raw material), different methods are adopted to remove iron. When the content of iron in the crude boric acid is greater than or equal to 0.001%, the crude boric acid, water and hydrogen peroxide are mixed, oxidation reaction is conducted to obtain an oxidation reaction solution with the boric acid concentration of less than 180g / L, heat preservation flocculation treatment is conducted on the oxidation reaction solution at the temperature of 65 DEG C or more for 30 minutes or more, and then solid-liquid separation and cooling crystallization are conducted. When the iron content in the crude boric acid is less than or equal to 0.0015%, after high-concentration crystallization of boric acid, a produced mother liquor and hydrogen peroxide are mixed for oxidation reaction, thus the mother liquor is recycled after flocculation and filtration, so that the iron in the system is maintained at a low balance level, and the product quality is ensured. The method provided by the invention breaks through the bottleneck that a recrystallization method has harsh requirements on the quality of raw materials, is simple in steps and low in cost, and is convenient for realizing large-scale production of high-purity boric acid products.
Owner:青海利亚达化工有限公司
Method for sequentially extracting chemical components in seawater
InactiveCN106044713ARealize comprehensive utilizationCalcium/strontium/barium carbonatesBromineSaline waterBromine
The invention discloses a method for sequentially extracting chemical components in seawater. The method comprises the following steps: (1) removing carbonates in the seawater; (2) extracting Mg<2+> and Ca<2+> in the seawater to generate Mg(OH)2, CaSO4 and CaCO3 products; (3) concentrating and crystallizing to obtain NaCl; (4) extracting the crystallized KCl; (5) extracting bromine; (6) extracting boron in concentrated brine in the form of a boric acid precipitate, and extracting fluorine ions in the form of CaF2; and (7) when the LiCl in the concentrated brine is approximate to the saturated state, adding soda ash into the concentrated brine so as to crystallize and separate out LiCl in the form of Li2CO3. A condensation-type turbogenerator unit electricity-water-salt cogeneration technique is utilized to sequentially extract the chemical components in the seawater to implement seawater comprehensive utilization, thereby generating Mg(OH)2 (which can be converted into MgO), CaSO4, CaCO3, NaCl, KCl, bromine, CaF2 and other products.
Owner:潘庆光
Quaternary positive electrode material and preparation method and application thereof
ActiveCN113582253AImprove cycle lifeMaintain high nickel capacitySecondary cellsPositive electrodesLithiumNiobium
The invention provides a quaternary positive electrode material and a preparation method and an application thereof, and the preparation method comprises the following steps: (1) mixing a quaternary precursor, a niobium source, a boron source and a lithium source, and carrying out primary calcination to obtain a boron / niobium doped quaternary positive electrode material; and (2) washing the boron / niobium doped quaternary positive electrode material obtained in the step (1) with water, drying, mixing with boric acid, and carrying out secondary calcination to obtain the quaternary positive electrode material, wherein the chemical formula of the quaternary precursor in the step (1) is LiNixCoyMnzAl(1-x-y-z)O2(x is greater than or equal to 0.9 and less than 1, y is greater than 0 and less than 0.07, and z is greater than 0 and less than 0.03). According to the preparation method disclosed by the invention, the lattice structure is stabilized and the generation of microcracks is limited by adjusting the textured microstructure of the ultrahigh nickel positive electrode material through a niobium / boron co-doping mechanism, so that the cycle life of the ultrahigh nickel positive electrode material is prolonged.
Owner:SVOLT ENERGY TECHNOLOGY CO LTD
Method for recovering lithium and producing high-purity boric acid or borax
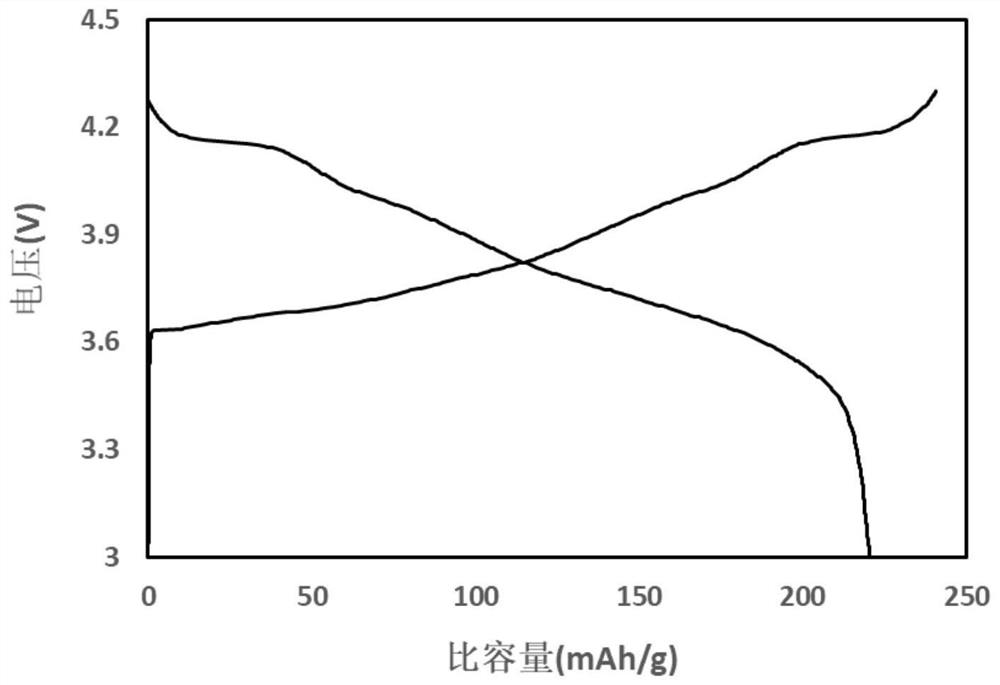
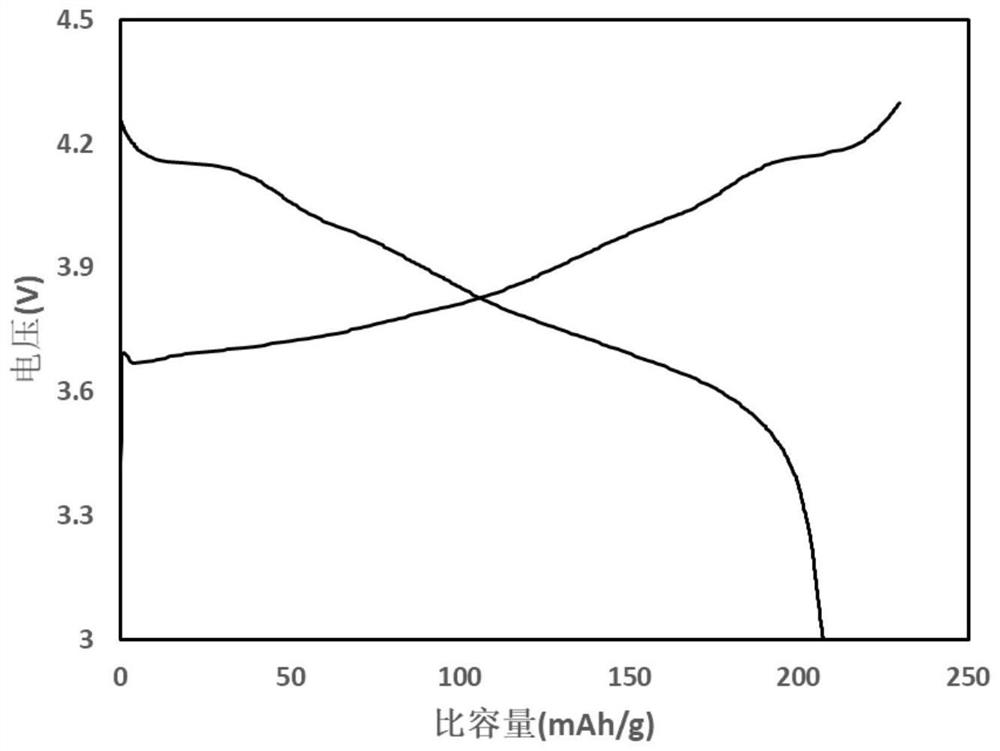
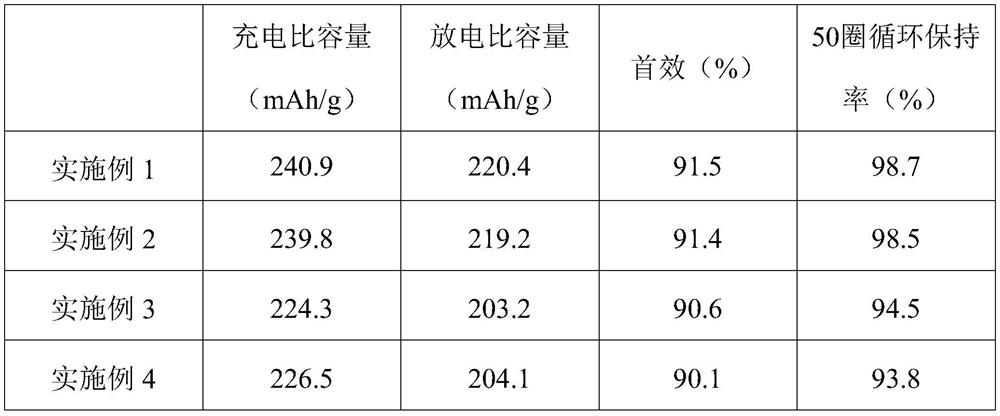
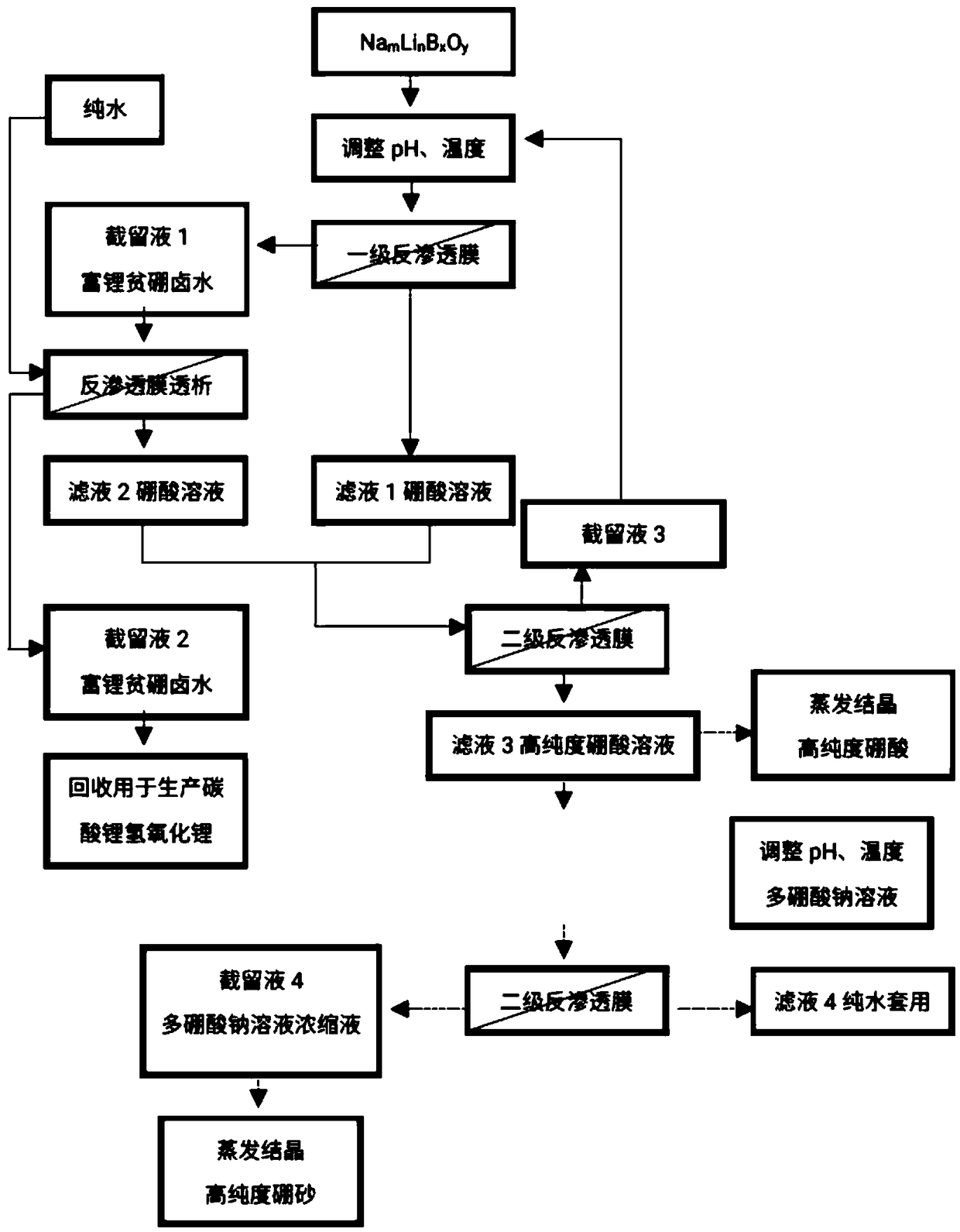
A method for recovering lithium and producing high-purity boric acid or borax comprises the steps that acid is added into a NamLinBxOy solution to adjust the pH to be 2.0-7.0, solution obtained afterpH adjustment is pumped into a membrane system, pressure is applied to the two sides of a reverse osmosis membrane to form a pressure difference, and part of water and H3BO3 in the solution are transferred from a high-voltage side to a low-pressure side through the membrane; lithium ions at the high-voltage side are enriched so as to obtain lithium-rich and poor-boron brine, and the aqueous solution on the low-pressure side is a boric acid solution containing a small quantity of lithium ions. The method is combined with a boron removal technology using a nanofiltration membrane method, the yield of the lithium ions is increased while boron elements in the brine are removed at low cost and at high efficiency, and comprehensive utilization of boron resources is achieved.
Owner:BEIJING QINGYUAN POWERISE TECH CO LTD +1
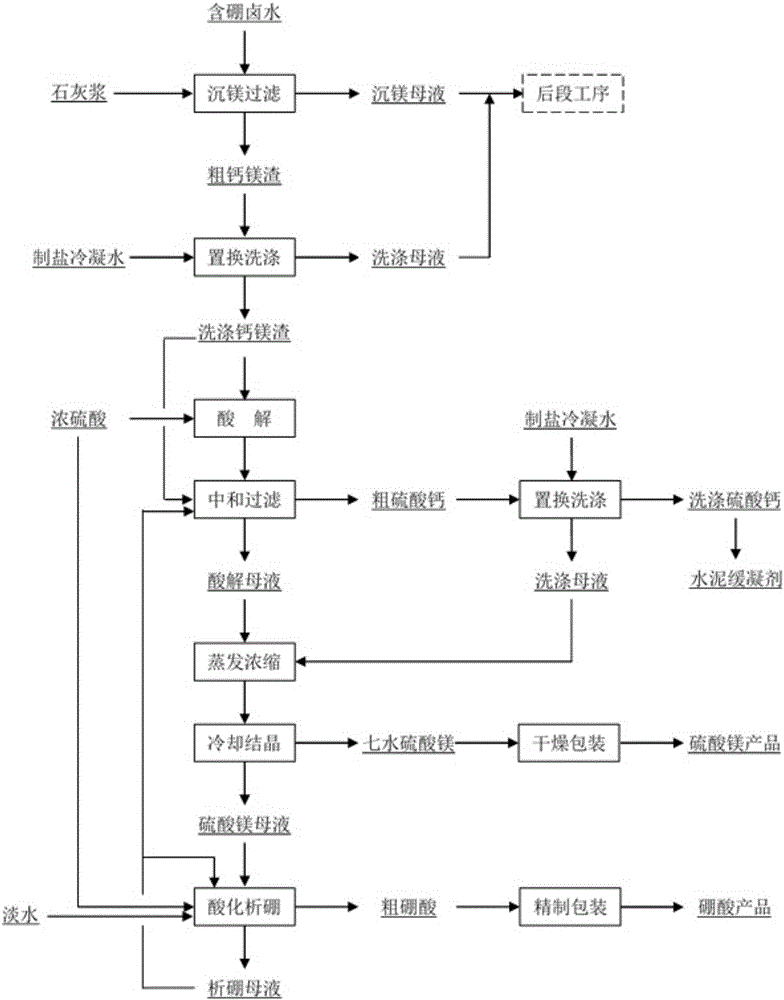
Method for recovering boron and magnesium from calcium magnesium dreg in boron-containing brine
ActiveCN106395844AExacerbated sinkingLow costCalcium/strontium/barium sulfatesMagnesium sulfatesSulfateBoron containing
The invention discloses a method for recovering boron and magnesium from a calcium magnesium dreg in boron-containing brine, and the method comprises the following steps: a) preparing a crude calcium magnesium dreg containing boron under suitable conditions, and washing the crude calcium magnesium dreg with a mother liquid displacement method to obtain a boron-containing washed calcium magnesium dreg; b) performing acidolysis on the boron-containing washed calcium magnesium dreg under acidic conditions to obtain a boron and magnesium-containing acidolysis solution and crude calcium sulfate; c) performing evaporation and concentration and cooling crystallization on the boron and magnesium-containing acidolysis solution to obtain an epsom salt product and magnesium precipitation mother liquor; and d)adding an acid into the magnesium precipitation mother liquor for acidification to obtain crude boric acid, and refining the crude boric acid to obtain a boric acid product. The processing method has the advantages of simple reaction principles of each step and easy operation processes, and can realize industrialized production.
Owner:四川恒成钾盐科技有限公司
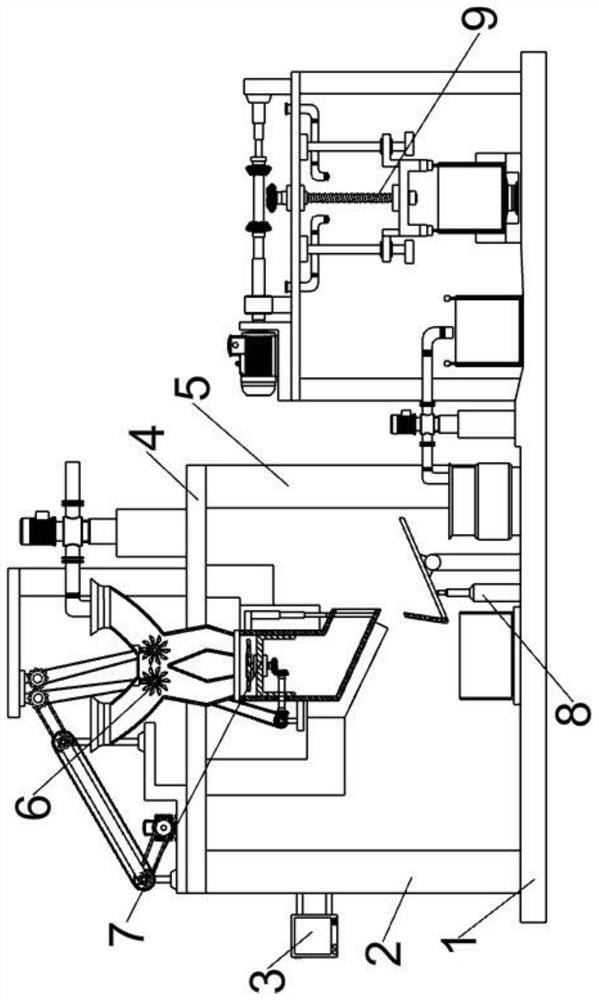
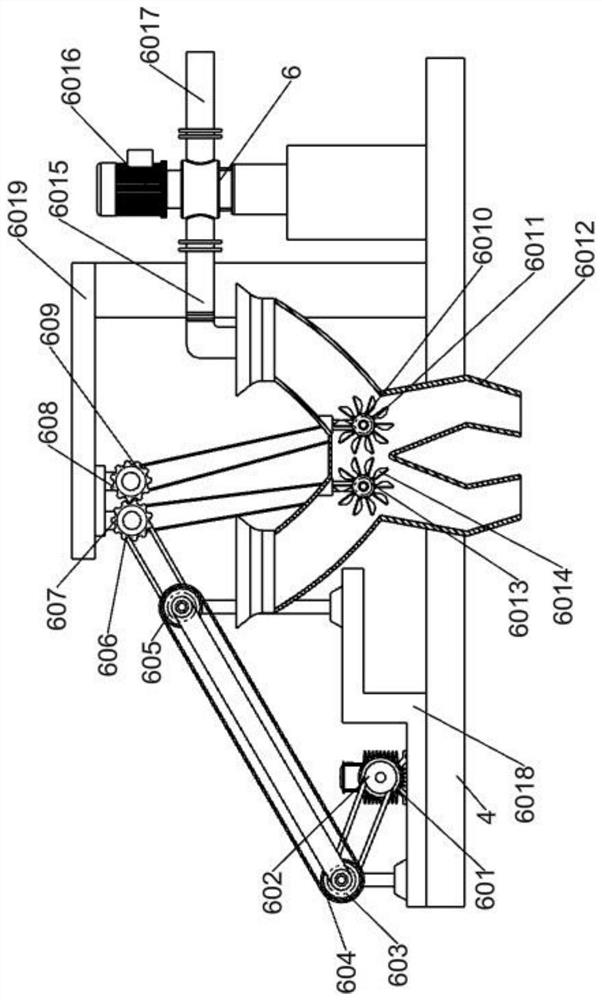
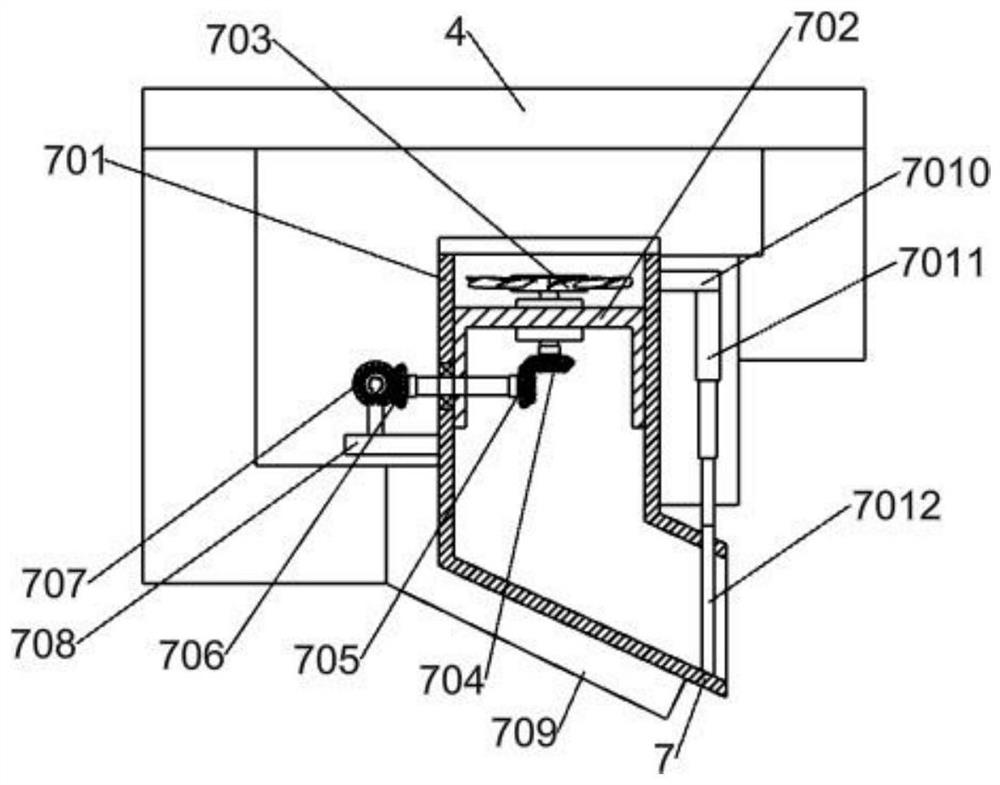
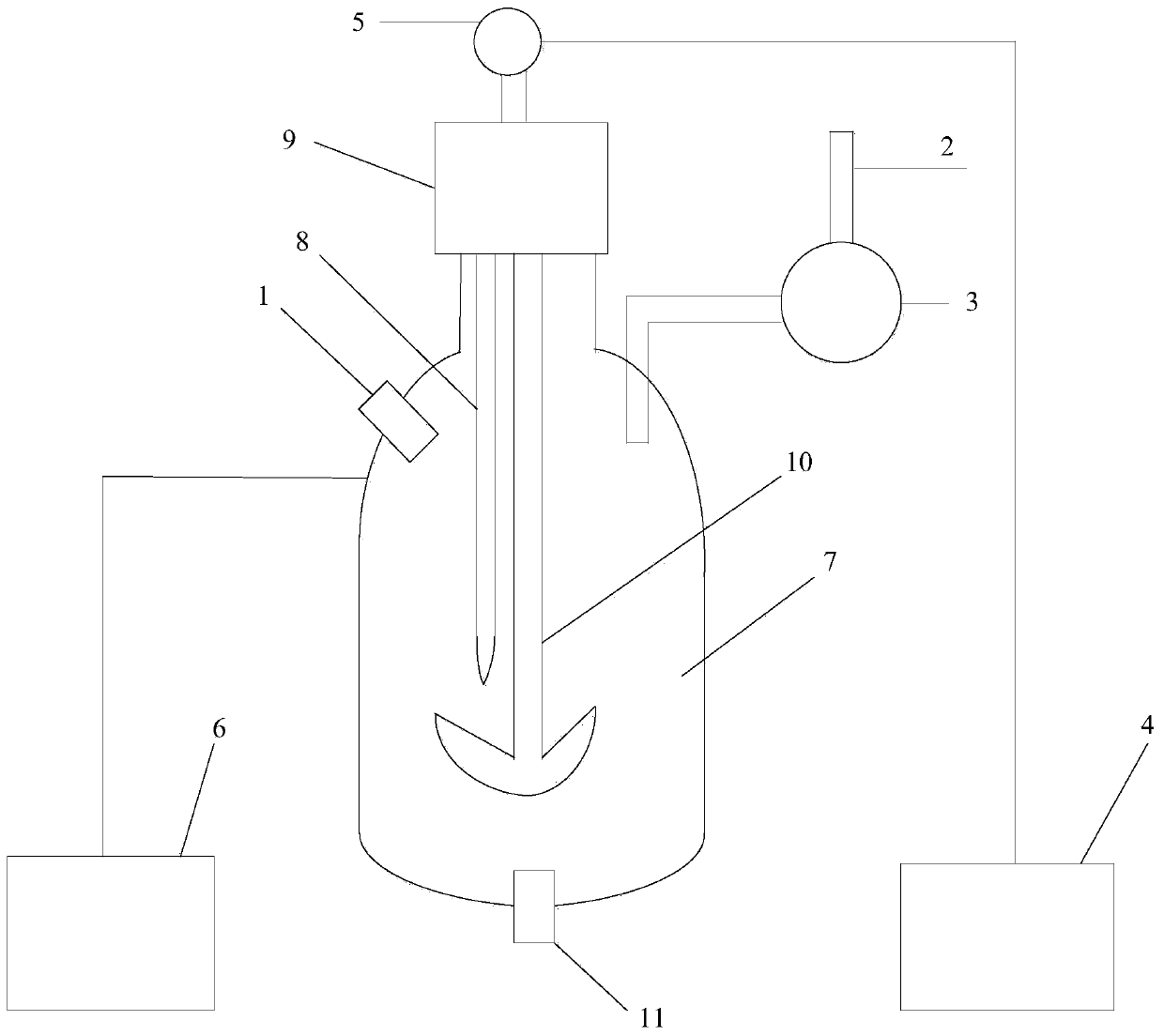
Refined boric acid preparation device for chemical engineering
The invention relates to the field of chemical engineering, in particular to a refined boric acid preparation device for chemical engineering. The technical problem to be solved by the invention is toprovide the refined boric acid preparation device for chemical engineering. The refined boric acid preparation device for chemical engineering comprises a working machine bottom plate, a second elevated column, a slag washing mechanism, a mixing and soaking mechanism, a filtering and hot melting mechanism, a boric acid precipitating and washing mechanism and the like, wherein the left side of thetop end of the working machine bottom plate is welded to a first elevated column. According to the invention, caked coarse boron slag is crushed and then is subjected to solid-liquid mixing again; sufficient soaking is conducted after mixing and washing, so magnesium chloride, magnesium sulfate and other impurities are fully dissolved and enter a liquid phase to be separated, and the use amount of a container is reduced; crystallized coarse boric acid is subjected to solid-liquid separation at the first time after solid precipitation is completed in the mode of crystallization separation viaa double-layer container; and suspended water spraying washing is conducted, so pure coarse boric acid is obtained.
Owner:李月珍
Method for separating and purifying boron-10 and boron-11
InactiveCN110143600AIncrease migration distanceShorten migration distanceIsotope separationBoron oxyacidsChromatographic separationIon exchange
The invention discloses a method for separating and purifying boron-10 and boron-11, belonging to the technical field of separation and preparation of isotopes. The method comprises the following three steps: the preparation of sample introduction liquid for a simulated moving bed; pretreatment of an ion exchange chromatographic column; and sample introduction and gradient elution. According to the method, the enrichment rate of <10>B can be increased to about 95% compared with a natural enrichment of 20%, and the speed of enrichment is fast; a simulated moving bed chromatographic separation system is used, so a long chromatographic column is not needed in the entire separation process, thereby reducing production cost; and gradient elution is utilized, so the purposes of reducing band migration distance and shortening separation period are achieved.
Owner:天津纯态化学工程技术有限公司
A kind of polarizing plate waste liquid treatment method
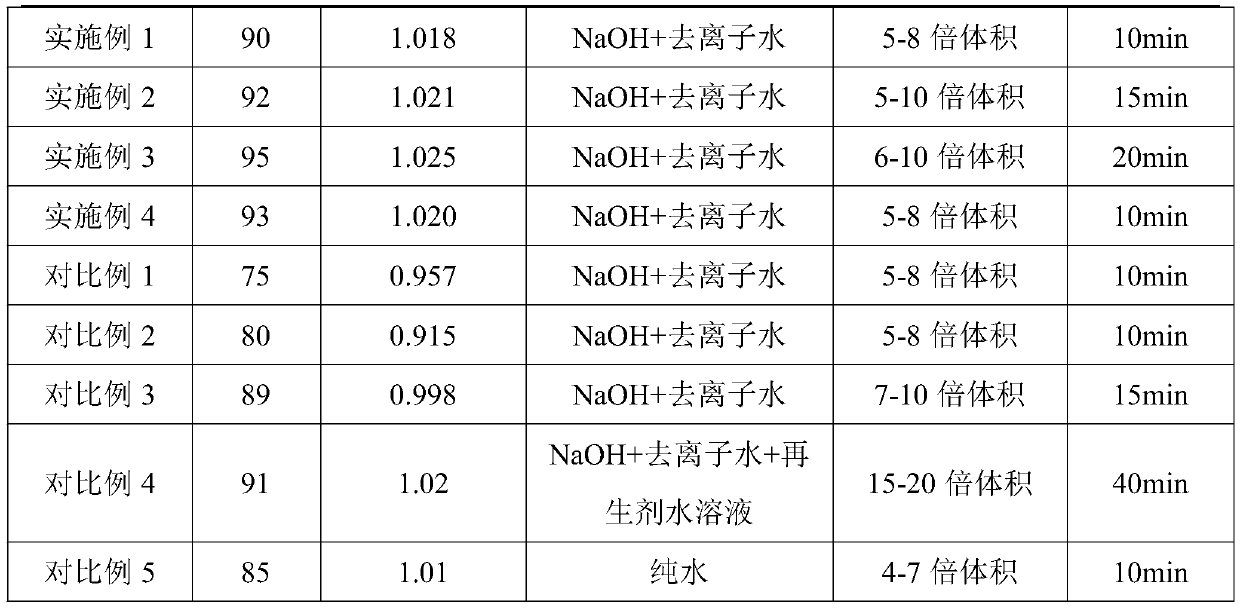
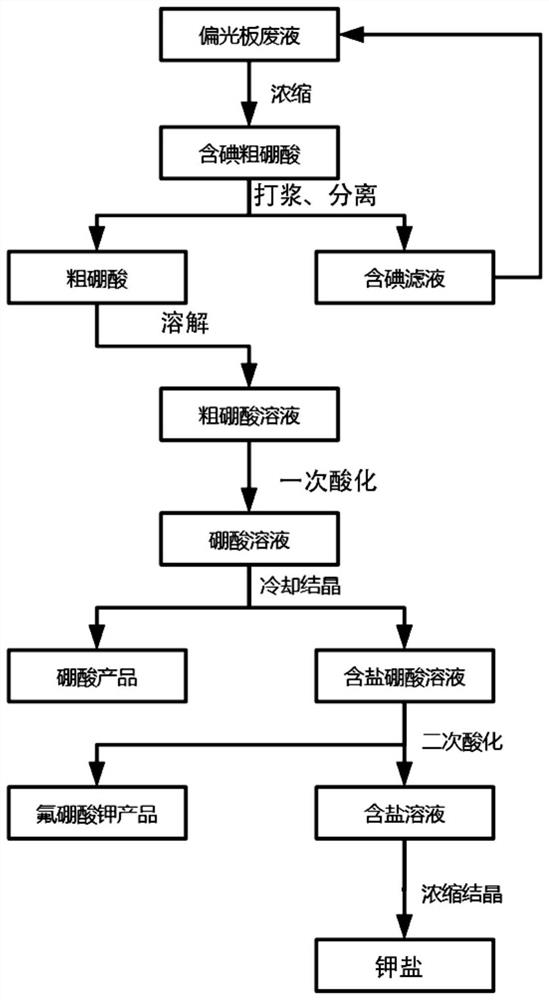
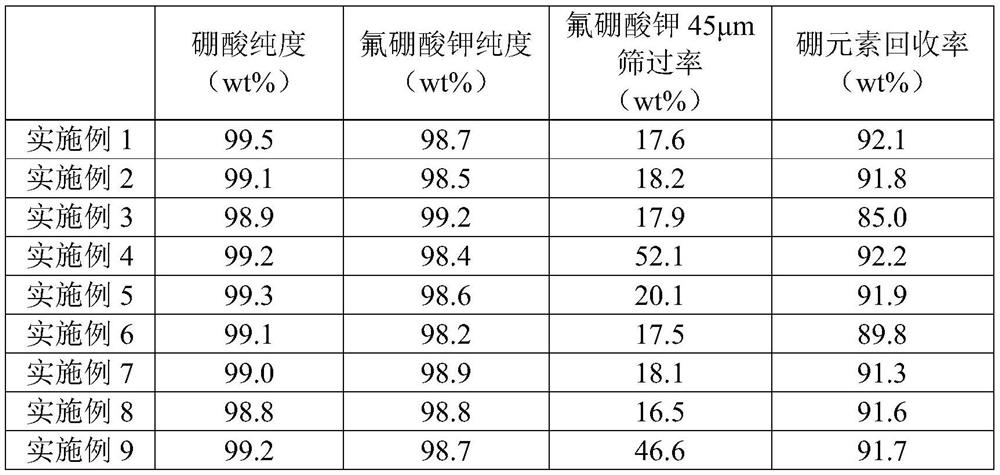
ActiveCN111498861BIncrease added valueHigh economic valueAlkali metal nitrate purificationTretrafluoboric acidFluoroboric acidSodium salt
The present invention provides a method for treating polarizing plate waste liquid. The method uses a hydrofluoric acid-containing solution to treat a salt-containing boric acid solution after pretreatment and primary acidification. The potassium fluoroborate product realizes the separation of boron element and inorganic potassium and sodium salts; and can use the hydrofluoric acid waste liquid produced by other industries to treat the salt-containing boric acid solution, which further reduces the waste liquid treatment cost and improves the economic value.
Owner:WUXI ZHONGTIAN SOLID WASTE DISPOSAL CO LTD
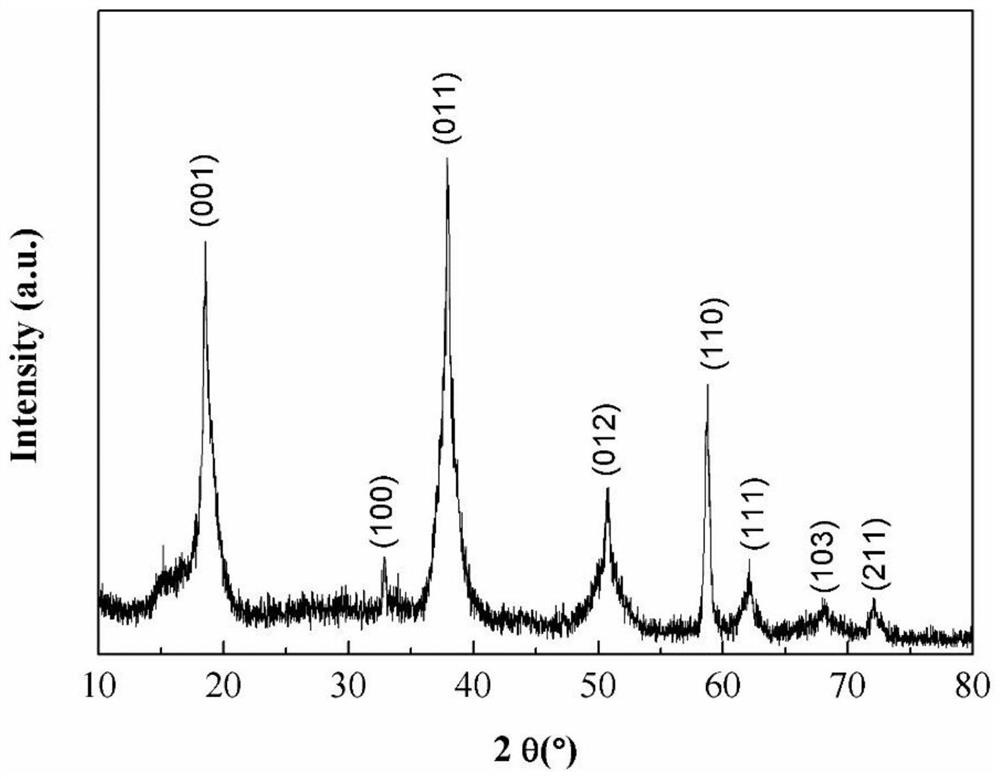
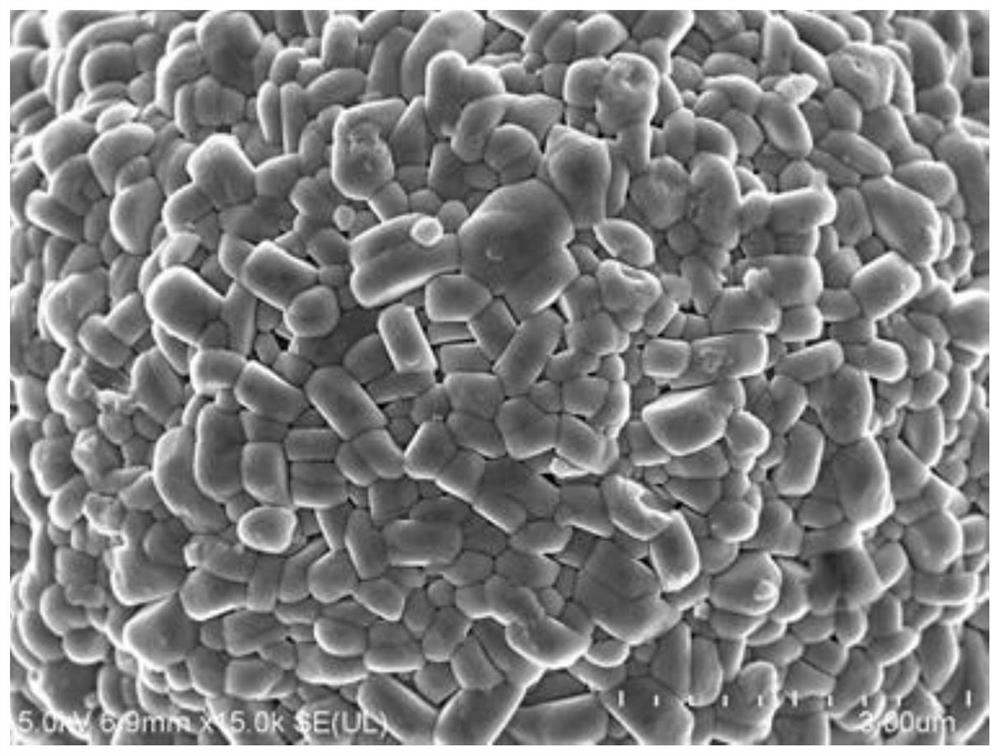
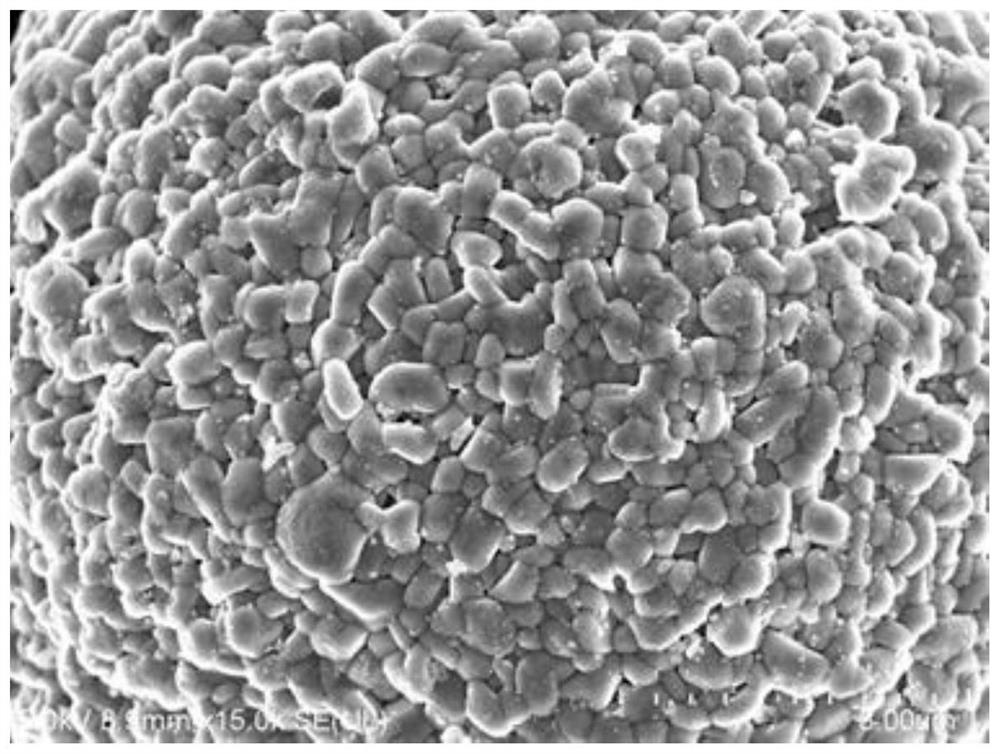
Method for preparing porous boric acid with coral structure
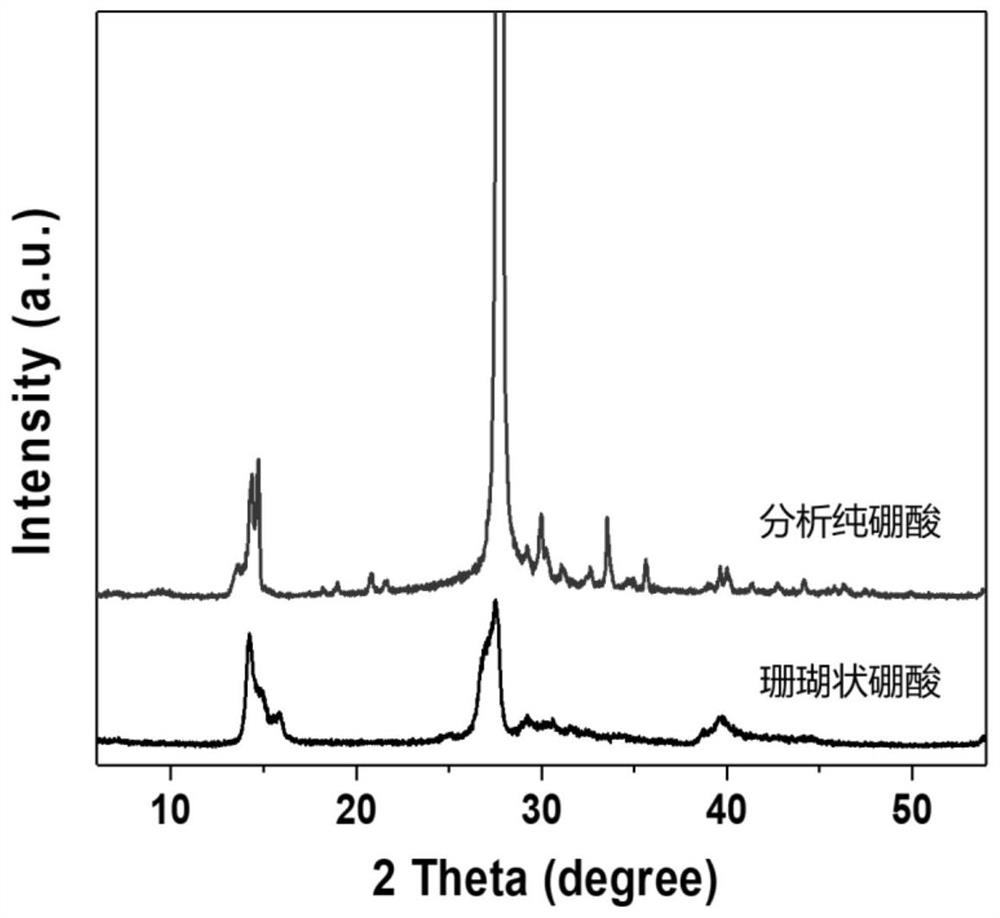
ActiveCN112174161ARecycling, environmental protection and savingHigh yieldBoron oxyacidsBoronic acidBiological materials
The invention belongs to the technical field of preparation of inorganic functional materials, and provides a method for preparing porous boric acid with a coral structure. According to the method, based on a coral reef structure forming mechanism in the ocean, cheap and easily-removed hydroxyl-containing organic molecules are selected as an inducer, and under the action of the organic induced molecules, the porous boric acid with the coral structure is obtained by changing the temperature gradient and the like in the dissolving, crystallizing and redissolving process. The method can realize uniform particle size of the boric acid product and safe and simple operation, and the porous structure can improve the surface reaction activity and the use efficiency. The method provides selectablematerial support for widening the application field of boric acid and improving the application performance of boric acid in the aspects of battery material and biological material design, catalysis and the like.
Owner:DALIAN UNIV OF TECH
Synthesis technology of tetrahydroxy diboron
ActiveCN108059169AYield increased significantlySafe and controllable processBoron oxyacidsBoronAqueous solution
The invention discloses a synthesis technology of tetrahydroxy diboron. The synthesis technology comprises the following steps: with tetraalkylamine diboron as a raw material, adding into an excess aqueous acid solution, reacting, then filtering, and performing vacuum drying to obtain the tetrahydroxy diboron. By the synthesis technology, operation is simple, the yield is high, and the product purity and the content reach 97% or above; the synthesis technology is suitable for industrial scale-up production.
Owner:CANGZHOU PURUI DONGFANG SCI & TECH
A kind of industrial production method of boron-10 acid and boron-11 acid
The invention relates to a method for industrially producing boron-10 acid and boron-11 acid from enriched boron-10 trifluoride and boron-11 trifluoride and solves the current problem that the boron-10 acid and the boron-11 acid are not good for industrial production. The method comprises the following steps: preparing a solid-liquid mixture of calcium carbonate and deionized water, heating the solid-liquid mixture, introducing boron-10 trifluoride or boron-11 trifluoride gas into a reaction kettle, and continuously stirring till the gas is uniformly dispersed in the solid-liquid mixture; performing suction filtration on the solid-liquid mixture; washing a filter cake by the deionized water, and mixing the washing solution with the mother solution; diluting the boric acid solution, rinsing and reducing the temperature, putting in a filter tank and continuously stirring; filtering the boric acid solution in the tank, reducing the temperature to room temperature and performing suction filtration again, putting the obtained boric acid solution into an evaporation tank to evaporate and condense, stopping dehydration when the solution is in a saturated mode, reducing the temperature, crystallizing, filtering and drying to obtain the boric acid crystal, namely boron-10 acid and boron-11 acid solid. The method is suitable for the industrial production.
Owner:浙江创世雷博科技有限公司
Nuclear-purity-grade boric acid standard substance preparation device
ActiveCN110817897ASimple structurePreparing sample for investigationChemical industryPhysical chemistryHeating system
The invention relates to the technical field of boric acid standard substance preparation, and particularly discloses a nuclear-purity-grade boric acid standard substance preparation device, wherein heat-conducting oil in an oil bath pot is pumped into a glass interlayer of a kettle body through a pressurizing pump in a heating system to fill the interlayer with the heat-conducting oil, and the whole kettle body is heated through the hot heat-conducting oil. According to the invention, the device is simple in structure and can be used for preparing boric acid standard substances, and the technical indexes of the prepared standard substance meet related requirements.
Owner:BEIJING RESEARCH INSTITUTE OF CHEMICAL ENGINEERING AND METALLURGY
Method for preparing boron fertilizer
InactiveUS8946120B2Low production costShorten production timeBiocideAmmonium nitratesDissolutionCooling down
A method for preparing a boron fertilizer, including: (1) heating boric acid to a temperature of 180-200° C., maintaining the temperature for 20-30 min for dehydration of the boric acid to yield pyroboric acid; and (2) cooling down the pyroboric acid to a temperature of 40-60° C., crushing, and screening to yield a powdered, weakly acidic, high-content boron fertilizer. The method is energy-saving, environmentally friendly, and low in cost. The resulting boron fertilizer is weakly acidic, fast in dissolution rate, and has excellent in compounding performance
Owner:UKHAN FISHERI EHGRIKALCHERAL SAJENS EHND TECH KO
Method for preparing secondary element water-soluble fertilizer by using mother liquor water-free method
PendingCN112723933AImprove product qualityIncrease contentCalcareous fertilisersMagnesium fertilisersAgricultural scienceAgricultural engineering
The invention discloses a method for preparing a secondary element water-soluble fertilizer by using a mother liquor water-free method, the fertilizer is a full water-soluble secondary element water-soluble fertilizer, and the production method mainly uses a water-soluble magnesium fertilizer, water-soluble calcium and a mother liquor as adhesives to prepare the secondary element water-soluble fertilizer. Compared with the prior art, the method has the advantages that the mother liquor is used as an adhesive to replace the traditional water granulation process, the over-high moisture content of the product caused by the original water granulation process is reduced, the drying link is omitted, the energy consumption is reduced, the problem that the product is easy to harden is solved, and the mother liquor granulation enables medium and trace elements contained in the mother liquor to further improve the related content index of the product; meanwhile, the fertilizer has the advantages of high nutrient content, complete nutrition, good water solubility, high fertilizer efficiency and high utilization rate. The utilization rate of boron, magnesium and calcium can reach more than 40%.
Owner:辽宁华昇金玛生态工程有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com