Preparation method of high-purity boric acid
A technology of high-purity boric acid and boric acid, which is applied in the direction of boron oxyacids and boron oxide compounds, can solve problems such as heat loss, high production cost, and increased raw material loss, and achieve reduced surface tension, reduced production costs, and shortened process time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
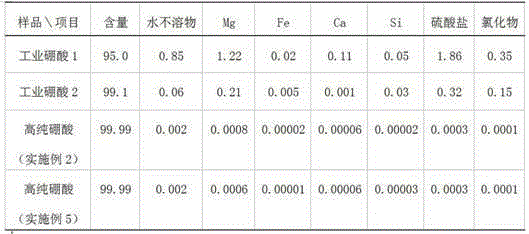
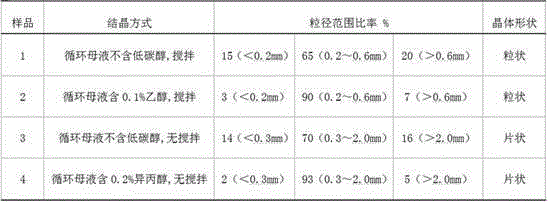
[0023] At room temperature, dissolve 4g of boric acid in 95.8g of deionized water, then add 0.2g of isopropanol to form 100g of initial circulating mother liquor, put it in a 500ml three-necked flask, then add 30g of industrial boric acid and 0.0006mol of sulfuric acid, stir and heat to 95 ℃, keep warm for 1 hour, stir and cool down to crystallize to room temperature, precipitate wet boric acid crystals, place them in a Buchner funnel, vacuum filter the mother liquor, the mother liquor passes through the cation exchange resin column and the anion exchange resin column in turn, and the obtained clean mother liquor is divided into two Wash the above-mentioned boric acid crystals, and quickly filter out the circulating mother liquor. The drained boric acid is placed in a vacuum drying oven and dried in vacuum at 70° C. for 3 hours to obtain 29 g of granular high-purity boric acid. The circulating mother liquor can be recycled repeatedly.
Embodiment 2
[0025] Add 60g of 95% industrial boric acid into a 1000ml three-necked flask, and 600g of boric acid saturated solution prepared at room temperature as the circulating mother liquor. The circulating mother liquor contains 0.6g of ethanol, then add 0.0012mol of hydrofluoric acid aqueous solution, stir and heat to 80°C, and keep warm for 2 hour, stirring and cooling down to crystallization to room temperature, separating out wet boric acid crystals and placing them in a Buchner funnel, vacuum-filtering the mother liquor, the mother liquor passing through a cation exchange resin column and an anion exchange resin column successively, and washing the boric acid crystals twice with the obtained clean mother liquor, and The circulating mother liquor was quickly filtered out, and the drained boric acid was placed in a vacuum drying oven, and vacuum-dried at 70° C. for 3 hours to obtain 55 g of granular high-purity boric acid.
Embodiment 3
[0027] Add 60g of industrial boric acid and 300g of the circulating mother liquor obtained from Example 2 into a 500ml three-necked flask, then add 0.001mol of nitric acid, stir and heat to 85°C, keep warm for 2 hours, stop stirring and naturally cool down to room temperature to obtain boric acid crystals, and wet boric acid The crystals are placed in a Buchner funnel, and the vacuum-filtered mother liquor passes through a cation exchange resin column and an anion exchange resin column in turn, and the obtained clean mother liquor washes the boric acid crystals twice, and quickly filters out the circulating mother liquor, and the dried boric acid crystals In a vacuum drying oven, vacuum-dry at 70°C for 3 hours to obtain 60 g of flaky high-purity boric acid, and the circulating mother liquor can be reused repeatedly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com