Method for preparing boric acid from ulexite
A technology of sodium borate and boric acid, which is applied in the field of inorganic chemistry and can solve problems such as the lack of methods for preparing boric acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Raw material composition analysis of sodium borate ore
[0037] Sodium borate ore is in powder form, and its composition content is as follows: B 2 o 3 32.94%, CaO 12.67%, MgO 1.03%, Cl 5.22%, moisture 16.73%.
[0038] Among them, the acid-insoluble content is 4%, and the approximate mineral composition is calculated as follows: NaCaB 5 o 9 ·8H 2 O content 76.6%, MgCl 2.44%, CaCO 3 3.75%, NaCl 5.61%, free water 7.6% (16.73%-7.6%=9.13% difference from the measured value, in fact, crystal water is released at 105°C or 110°C).
[0039] CaO in minerals is here referred to as NaCaB 5 o 9 ·8H 2 O and CaCO 3 In fact, it is also possible to calculate the existence of the form as CaCl 2 or CaSO 4 2H 2 The O form exists, but according to the bubble (colloid) situation, CaCO 3 Definitely the main form.
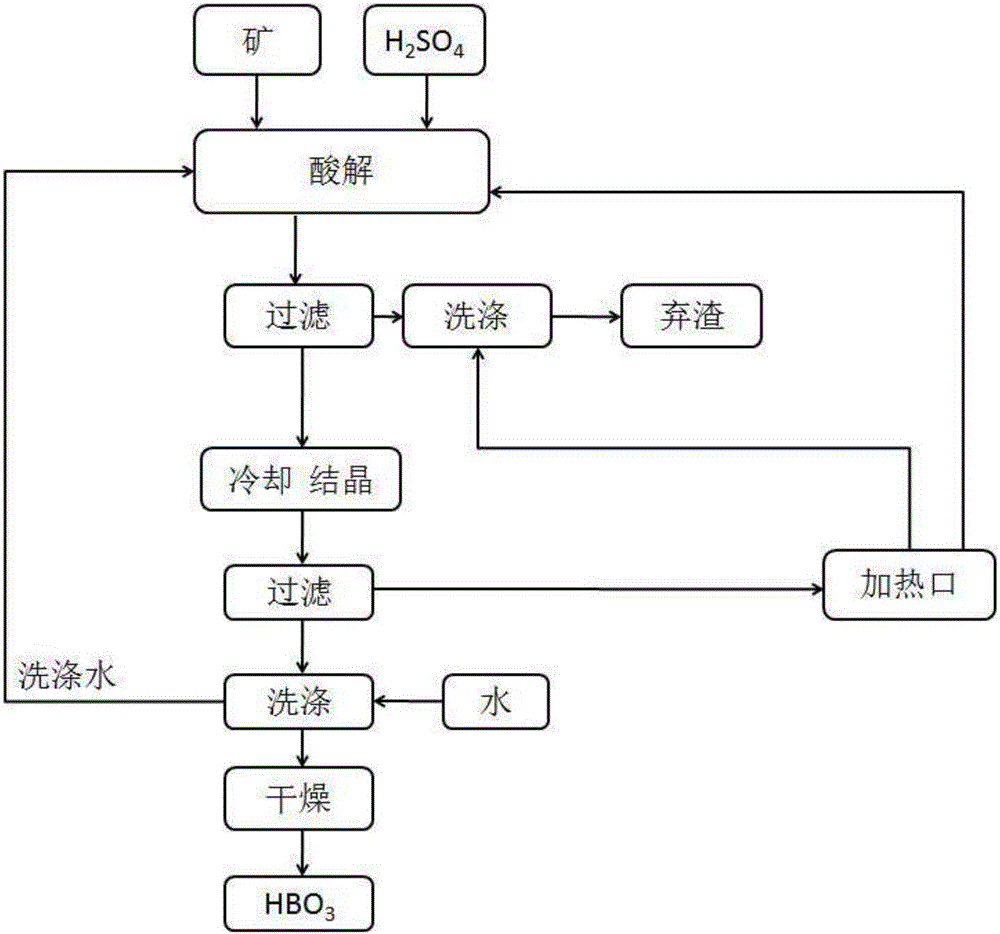
[0040]2, a kind of method that boric acid is prepared as raw material with sodium borate ore, comprises the following steps (technical process such as figure 1...
Embodiment 2
[0048] A method for preparing boric acid as raw material with sodium borate ore, comprising the following steps:
[0049] 1) Take powdery sodium borate ore and mix it with concentrated sulfuric acid, and react in the range of 95-105°C;
[0050] 2) solid-liquid separation to get the supernatant;
[0051] 3) cooling and crystallizing the supernatant described in step 2);
[0052] 4) solid-liquid separation to get precipitation;
[0053] 5) Take the precipitate described in step 4) and dry it to obtain the boric acid product.
[0054] On the basis of the above technical solutions, the following conditions are met:
[0055] The reaction temperature described in step 1) is 100°C.
[0056] In step 1), perform step 2) after the reaction is complete within the range of 95-105°C.
[0057] The reaction time in step 1) is 8-10 hours.
[0058] During the reaction in step 1), the mixture of powdery sodium borate ore and concentrated sulfuric acid is in a stirring state.
[0059] The...
Embodiment 3
[0065] A method for preparing boric acid as raw material with sodium borate ore, comprising the following steps:
[0066] 1) Take powdery sodium borate ore and mix it with concentrated sulfuric acid, and react in the range of 95-105°C;
[0067] 2) solid-liquid separation to get the supernatant;
[0068] 3) cooling and crystallizing the supernatant described in step 2);
[0069] 4) solid-liquid separation to get precipitation;
[0070] 5) Take the precipitate described in step 4) and dry it to obtain the boric acid product.
[0071] On the basis of the above technical solutions, the following conditions are met:
[0072] The reaction temperature described in step 1) is 100°C.
[0073] In step 1), perform step 2) after the reaction is complete within the range of 95-105°C.
[0074] The solid-liquid separation described in step 2) is to utilize plate and frame filter to filter.
[0075] Step 3) Adjust the pH during the cooling and crystallization process so that the pH of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com