Salicylic acid functionalized boron chelating adsorbent preparation method, product prepared therethrough, and application of product
A technology of chelating adsorbent and salicylic acid, which is applied in boron oxide compounds, chemical instruments and methods, boron oxyacids, etc., to achieve the effects of high-efficiency extraction, mild reaction conditions, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
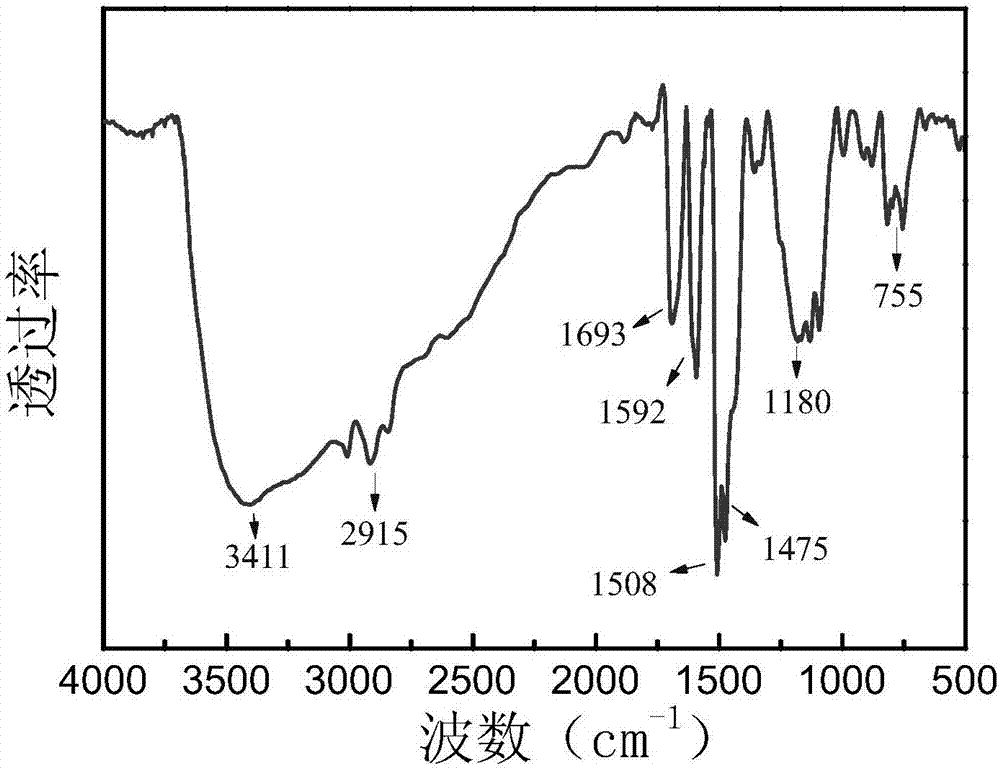
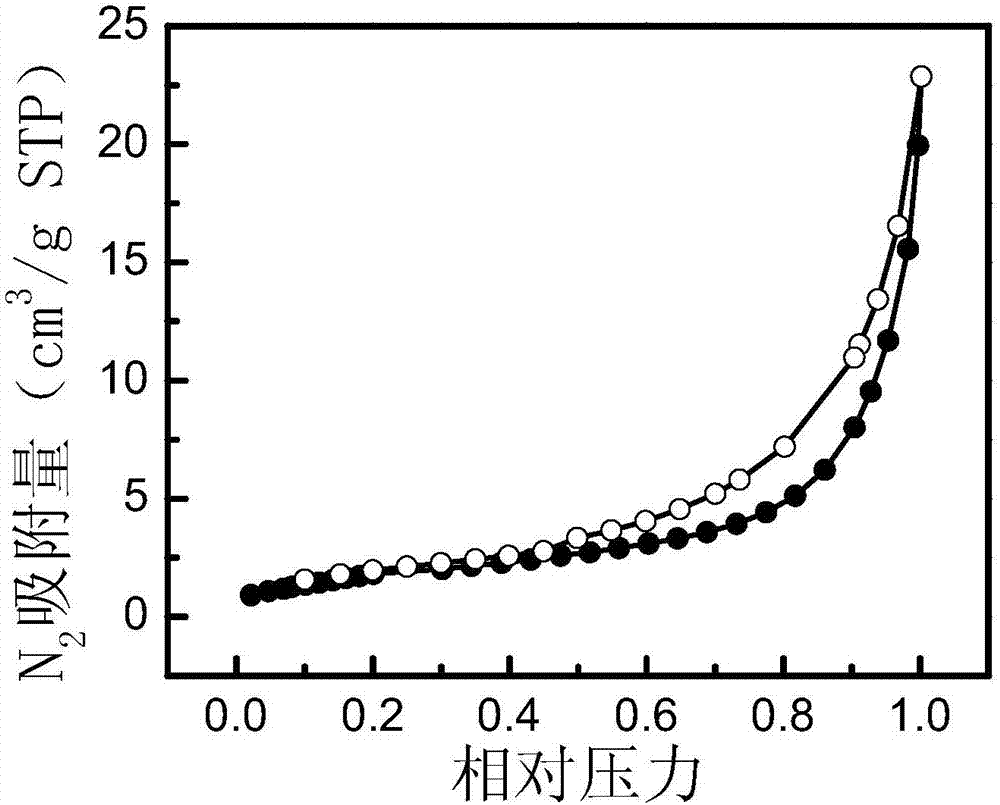
[0034] Preparation of salicylic acid-functionalized boron chelate adsorbent: 2.00 g of salicylic acid, 1.36 g of phenol, 3.53 g of formaldehyde, 0.2 mL of concentrated hydrochloric acid, and 7 mL of ethylene glycol methyl ether were stirred and dissolved to prepare an aqueous phase solution. Stir 1.00g of Span-80 and 100mL of liquid paraffin evenly to obtain an oil phase solution. After the aqueous phase solution was pre-polymerized at 65°C for 2h, it was slowly added to the oil phase, and reacted at a mechanical stirring rate of 380rpm and 90°C for 10h to obtain the primary product. The initial product was subjected to Soxhlet extraction with acetone for 48 hours to remove impurities such as porogens and reaction raw materials, and finally the obtained adsorbent was vacuum-dried at 50° C. for 12 hours to obtain the final product.
[0035] According to the test, the time for this product to reach adsorption equilibrium in 1000 mg / L boric acid aqueous solution at 25°C and pH 9 ...
Embodiment 2
[0038] Preparation of catechol functionalized boron chelate adsorbent: 1.59g catechol, 1.36g phenol, 3.53g formaldehyde, 0.2mL concentrated hydrochloric acid, 7mL ethylene glycol methyl ether were stirred and dissolved to prepare an aqueous phase solution. Stir 1.00g of Span-80 and 100mL of liquid paraffin evenly to obtain an oil phase solution. After the aqueous phase solution was pre-polymerized at 65°C for 2h, it was slowly added to the oil phase, and reacted at a mechanical stirring rate of 380rpm and 90°C for 10h to obtain the primary product. The initial product was subjected to Soxhlet extraction with acetone for 48 hours to remove impurities such as porogens and reaction raw materials, and finally the obtained adsorbent was vacuum-dried at 50° C. for 12 hours to obtain the final product.
[0039] After testing, the product reaches adsorption equilibrium in 1000mg / L boric acid aqueous solution for 30 minutes at 25°C and pH 9, and the saturated adsorption capacity can re...
Embodiment 3
[0041] Preparation of 2,5-dihydroxy-1,4-benzenedicarboxylic acid functionalized boron chelate adsorbent: 2.87g 2,5-dihydroxy-1,4-benzenedicarboxylic acid, 1.36g phenol, 3.53g formaldehyde , 0.2mL of concentrated hydrochloric acid, and 7mL of ethylene glycol methyl ether were stirred and dissolved to obtain an aqueous phase solution. Stir 1.00g of Span-80 and 100mL of liquid paraffin evenly to obtain an oil phase solution. After the aqueous phase solution was pre-polymerized at 65°C for 2h, it was slowly added to the oil phase, and reacted at a mechanical stirring rate of 380rpm and 90°C for 10h to obtain the primary product. The initial product was subjected to Soxhlet extraction with acetone for 48 hours to remove impurities such as porogens and reaction raw materials, and finally the obtained adsorbent was vacuum-dried at 50° C. for 12 hours to obtain the final product.
[0042] After testing, the product reaches adsorption equilibrium in 1000mg / L boric acid aqueous solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com