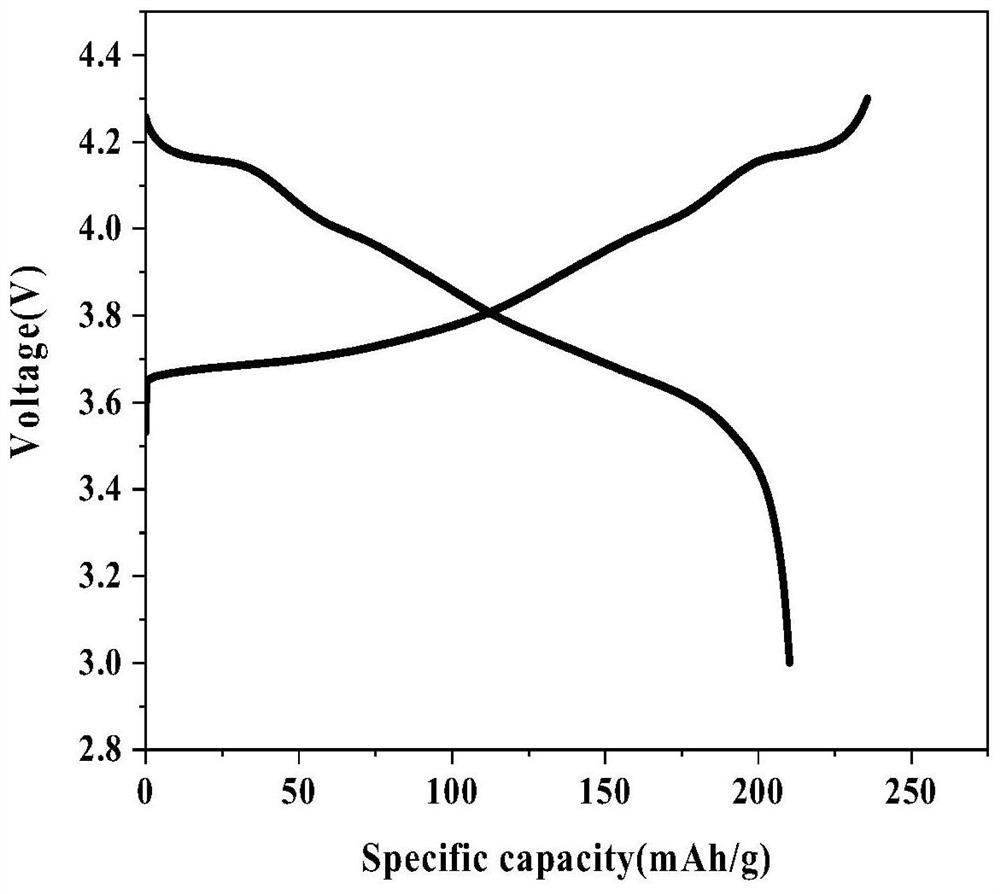
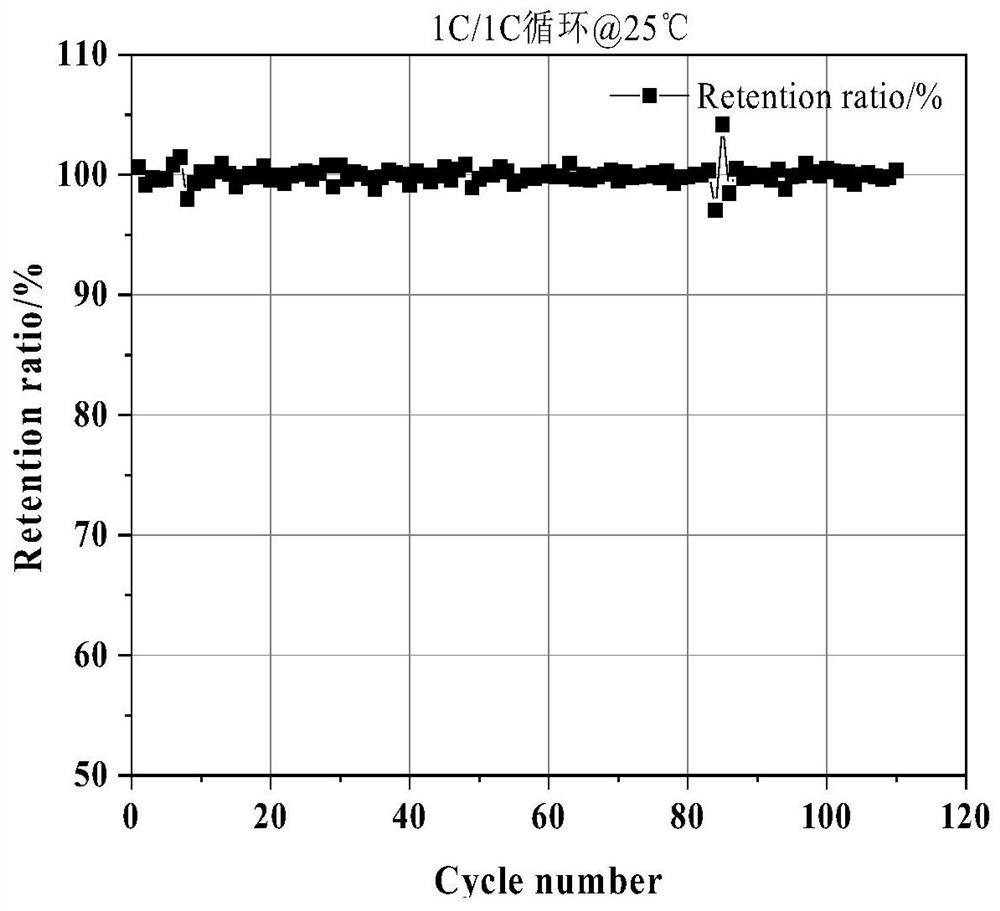
Lithium iron phosphate-boric acid co-coated lithium nickel cobalt aluminate positive electrode material and preparation method thereof
A technology of lithium nickel cobalt aluminate and positive electrode materials, which is applied in the direction of positive electrodes, chemical instruments and methods, nickel compounds, etc., can solve problems such as cycle performance and safety performance of lithium nickel cobalt aluminate, and achieve improved cycle performance and safety Performance, improvement of electrical conductivity, and improvement of processability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] A preparation method of lithium iron phosphate-boric acid co-coated nickel cobalt lithium aluminate positive electrode material, comprising the following steps:
[0027] Step 1: Weigh the nickel-cobalt-lithium-aluminate precursor, mix it with lithium and sinter it to obtain the primary sintered material of nickel-cobalt-lithium aluminate; the lithium source used when mixing lithium is lithium hydroxide, and the nickel-cobalt-lithium aluminate precursor and The molar ratio of the lithium source is 1:(1.01-1.06), the sintering temperature is 650-750°C, and the holding time is 9-15hh;
[0028] Step 2: Add the primary sintered material of nickel cobalt lithium aluminate into deionized water for stirring and washing, the mass ratio of deionized water to the primary sintering material of nickel cobalt lithium aluminate is (1-5):1, and the stirring and washing time is 10- 60min, the stirring line speed is 500rpm, vacuum drying after solid-liquid separation to obtain matrix pos...
Embodiment 1
[0031] Step 1: Weigh a certain mass of nickel cobalt lithium aluminate precursor, the molecular formula is Ni 0.91 co 0.06 Al 0.03 (OH) 2 , mixed lithium hydroxide (LiOH·H 2 After O) sintering is carried out to obtain the primary sintered material of nickel cobalt lithium aluminate. The molar ratio of nickel-cobalt-lithium-aluminate precursor to lithium source is 1:1.01, the sintering temperature is 650°C, and the holding time is 9h.
[0032] Step 2: Weigh a certain mass of nickel-cobalt lithium aluminate primary sintering material, add a certain mass of deionized water according to the water-to-material ratio of 1:1, and perform stirring and washing. The stirring speed is 500 rpm, and the washing time is 10 minutes. Afterwards, it was suction filtered and vacuum dried for 4 hours at a temperature of 100° C. to obtain a nickel-cobalt-lithium-aluminate cathode material matrix.
[0033] Step 3: Ball milling and mixing the base positive electrode material and the coating lit...
Embodiment 2
[0036] Step 1: Weigh a certain mass of nickel cobalt lithium aluminate precursor, the molecular formula is Ni 0.91 co 0.06 Al 0.03 (OH) 2, mixed lithium hydroxide (LiOH·H 2 After O) sintering is carried out to obtain the primary sintered material of nickel cobalt lithium aluminate. The molar ratio of nickel-cobalt-lithium-aluminate precursor to lithium source is 1:1.02, the sintering temperature is 710°C, and the holding time is 12h.
[0037] Step 2: Weigh a certain mass of nickel-cobalt lithium aluminate primary sintering material, add a certain mass of deionized water according to the water-to-material ratio of 2:1 to carry out stirring and washing, the stirring speed is 500rpm, and the washing time is 30min. Afterwards, centrifuge and vacuum dry for 6 hours at a temperature of 120° C. to obtain a nickel-cobalt-lithium-aluminate cathode material matrix.
[0038] Step 3: Mix the matrix positive electrode material and the coating lithium iron phosphate-boric acid through ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge ratio | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com