Boric acid composite extractant and method for recovering boric acid, magnesium and lithium from salt lake old brine
A compound extractant, the technology of salt lake old brine, applied in lithium carbonate;/acid carbonate, magnesium carbonate, boron oxyacid, etc., can solve the problem of unsatisfactory extraction rate, high energy consumption, and dosage of chemicals Large and other problems, to achieve the effect of high-value recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
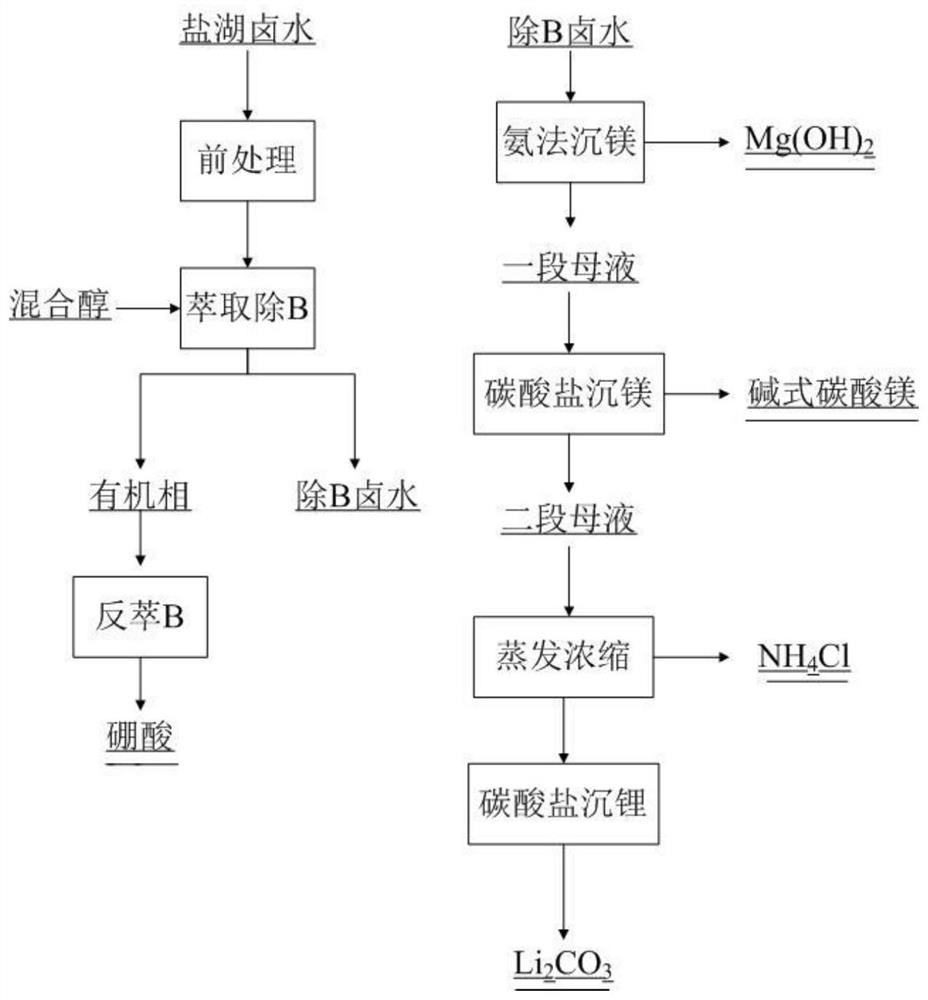
[0055] (1) the old brine of the salt lake is acidified with hydrochloric acid to adjust the pH value to 1.25, then adopt 20% mixed alcohol (isooctanol, isoamyl alcohol, 2-ethyl-1,3 hexanediol mixed alcohol (mass ratio 1:1:1) extraction, 80% sulfonated kerosene as diluent, control O / A to 1:1, after one-stage extraction of B, the extraction rate of B reaches 92.01%, back extraction with 0.1mol / L NaOH solution , O / A is 1:1, get H 3 BO 3 , after concentration and crystallization to obtain H 3 BO 3 product;
[0056] (2) Precipitate the brine after removing B with ammonia water, control the reaction temperature to 70°C, control the reaction pH to 8.0, and the reaction time is 2h, filter and wash to obtain Mg(OH) 2 Product, the recovery rate of Mg is 93.6%;
[0057] (3) Add ammonia water and ammonium bicarbonate to a section of the mother liquor of heavy magnesium, adjust the pH to 9.0, the amount of ammonium bicarbonate is 1.5 times the theoretical amount, control the reaction ...
Embodiment 2
[0060] (1) The old brine of the salt lake is acidified with hydrochloric acid to adjust the pH value to 1.25, and then 30% of mixed alcohol (isooctanol, isoamyl alcohol, 2-ethyl-1,3 hexanediol mixed alcohol (1: 1:1) extraction, 70% sulfonated kerosene as diluent, control O / A to 1:1, after one-stage extraction B, the extraction rate of B reaches 98.177%, back extraction with 0.1mol / L NaOH solution, O / A 1:1, get H 3 BO 3 , after concentration and crystallization to obtain H 3 BO 3 product;
[0061] (2) Precipitate the brine after removing B with ammonia water, control the reaction temperature to 70°C, control the reaction pH to 8.0, and the reaction time is 2h, filter and wash to obtain Mg(OH) 2 Product, the recovery rate of Mg is 93.6%;
[0062] (3) Add ammonia water and ammonium bicarbonate to a section of heavy magnesium mother liquor, adjust the pH to 9.0, the amount of ammonium bicarbonate is 1.5 times of the theoretical amount, control the reaction temperature at 60°C...
Embodiment 3
[0065] (1) The old brine of the salt lake is acidified with hydrochloric acid to adjust the pH value to 1.25, then adopt 40% mixed alcohol (the mixed alcohol of isooctyl alcohol, isoamyl alcohol, 2-ethyl-1,3 hexanediol (mass ratio 1:1:1)) extraction, 60% sulfonated kerosene as diluent, control O / A to 1:1, after primary extraction B, the extraction rate of B reaches 99%, back extraction with 0.1mol / L NaOH solution , O / A is 1:1, get H 3 BO 3 , after concentration and crystallization to obtain H 3 BO 3 product;
[0066] (2) Precipitate the brine after removing B with ammonia water, control the reaction temperature to 70°C, control the reaction pH to 8.0, and the reaction time is 2h, filter and wash to obtain Mg(OH) 2 Product, the recovery rate of Mg is 93.6%;
[0067] (3) Add ammonia water and ammonium bicarbonate to a section of heavy magnesium mother liquor, adjust the pH to 9.0, the amount of ammonium bicarbonate is 1.5 times of the theoretical amount, control the reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com