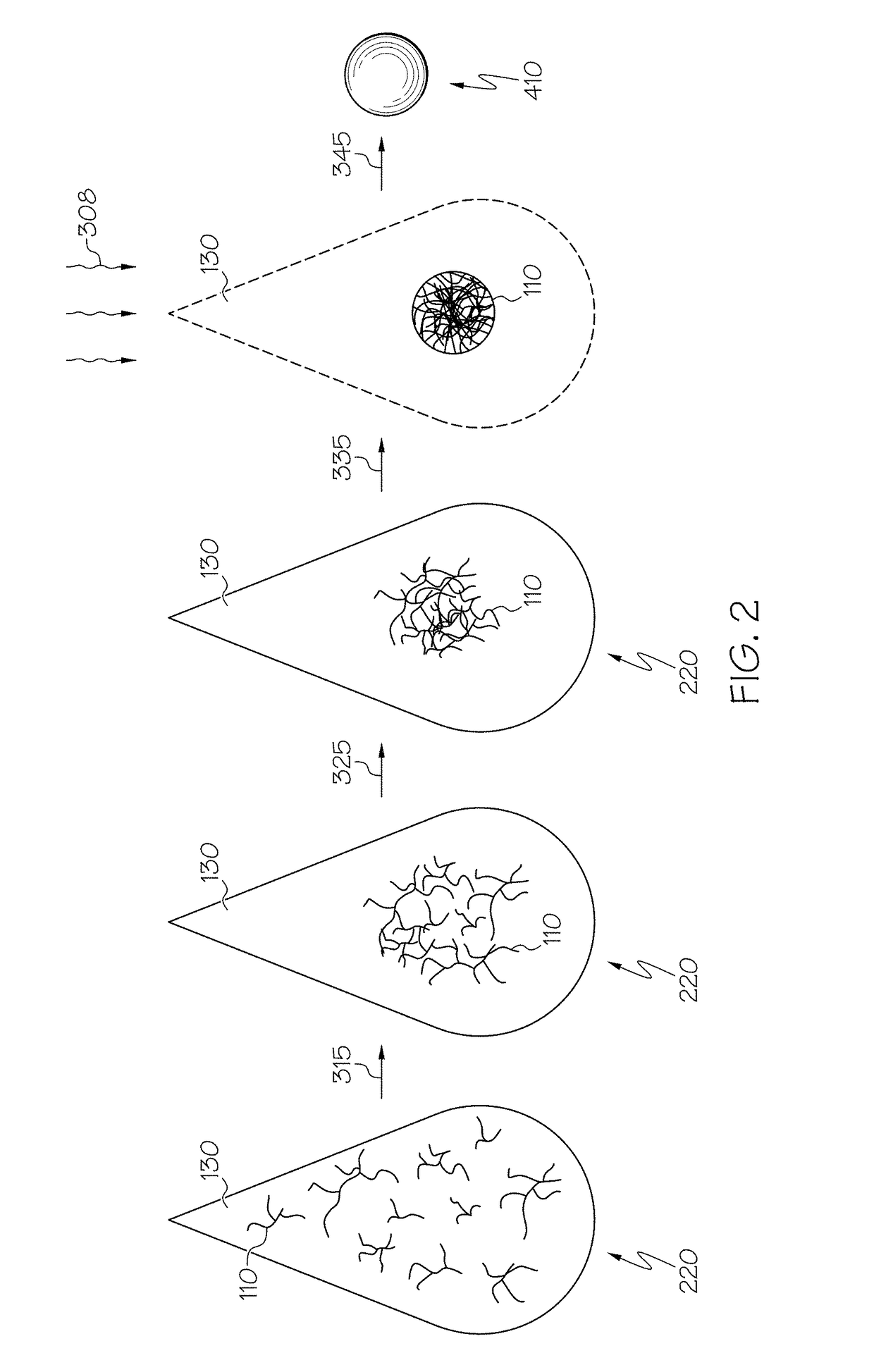
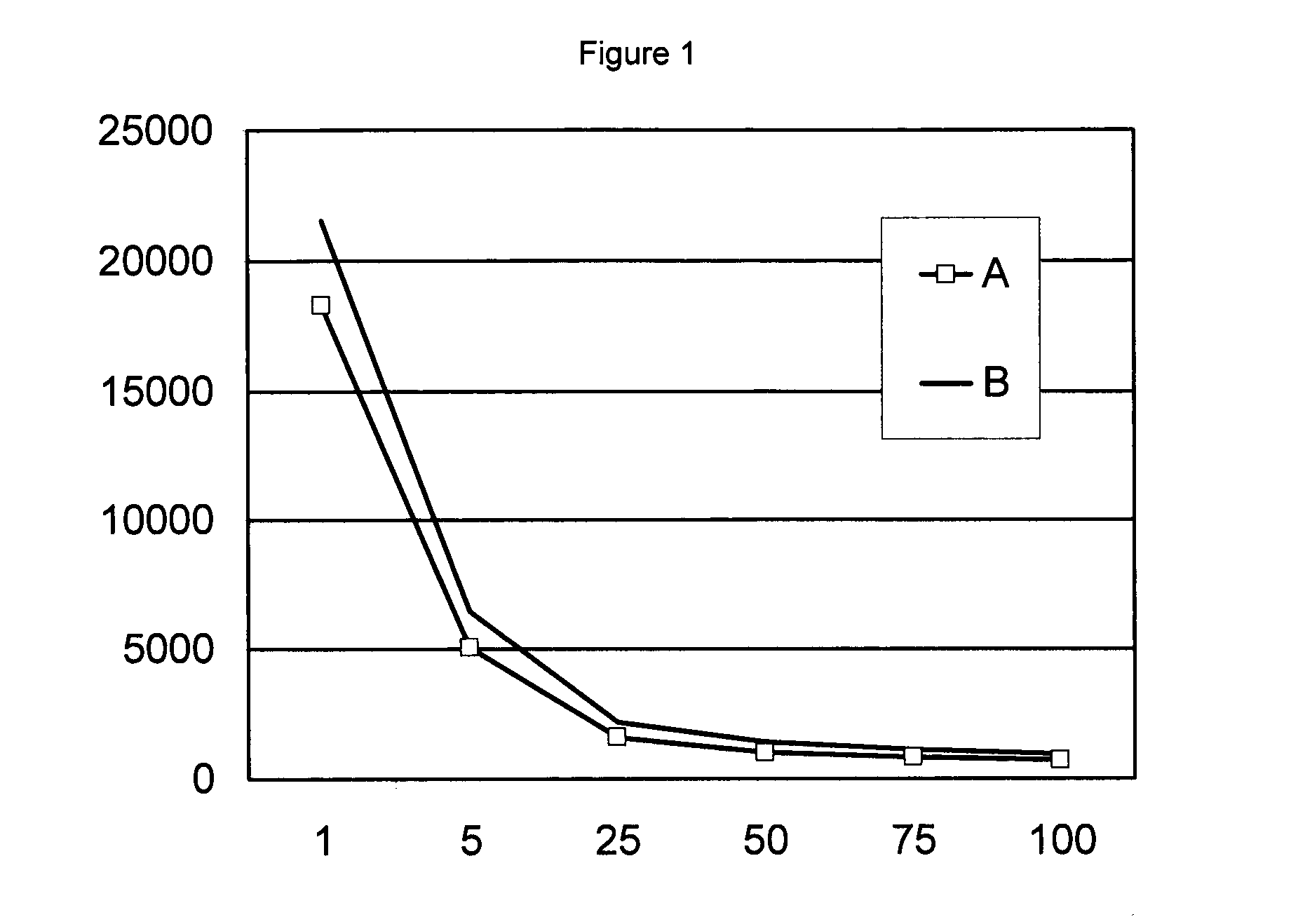
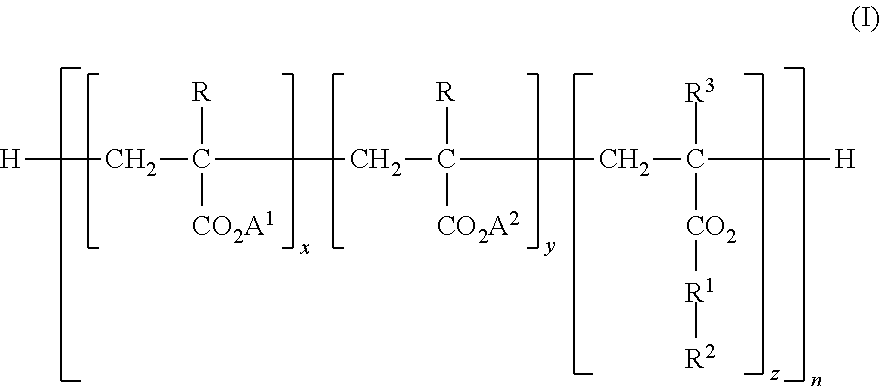
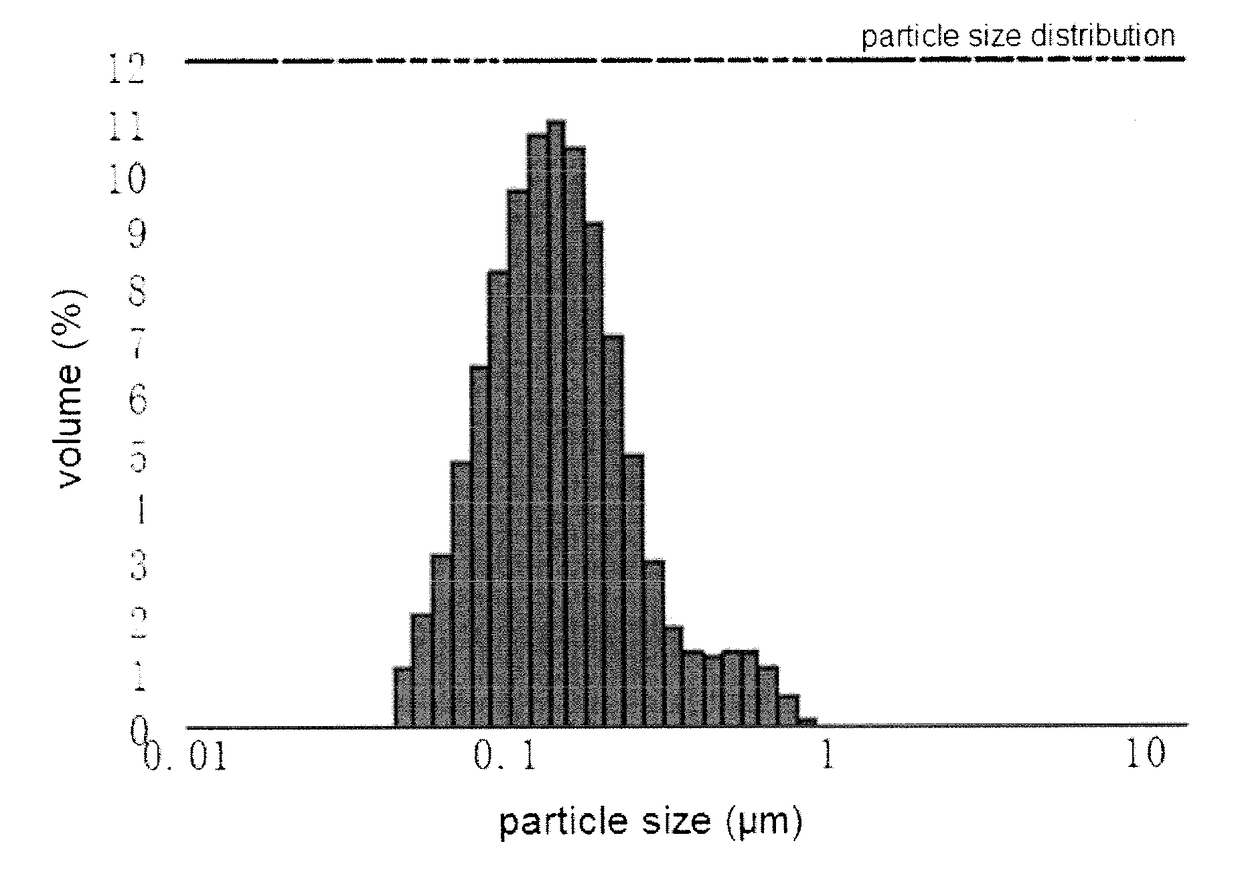
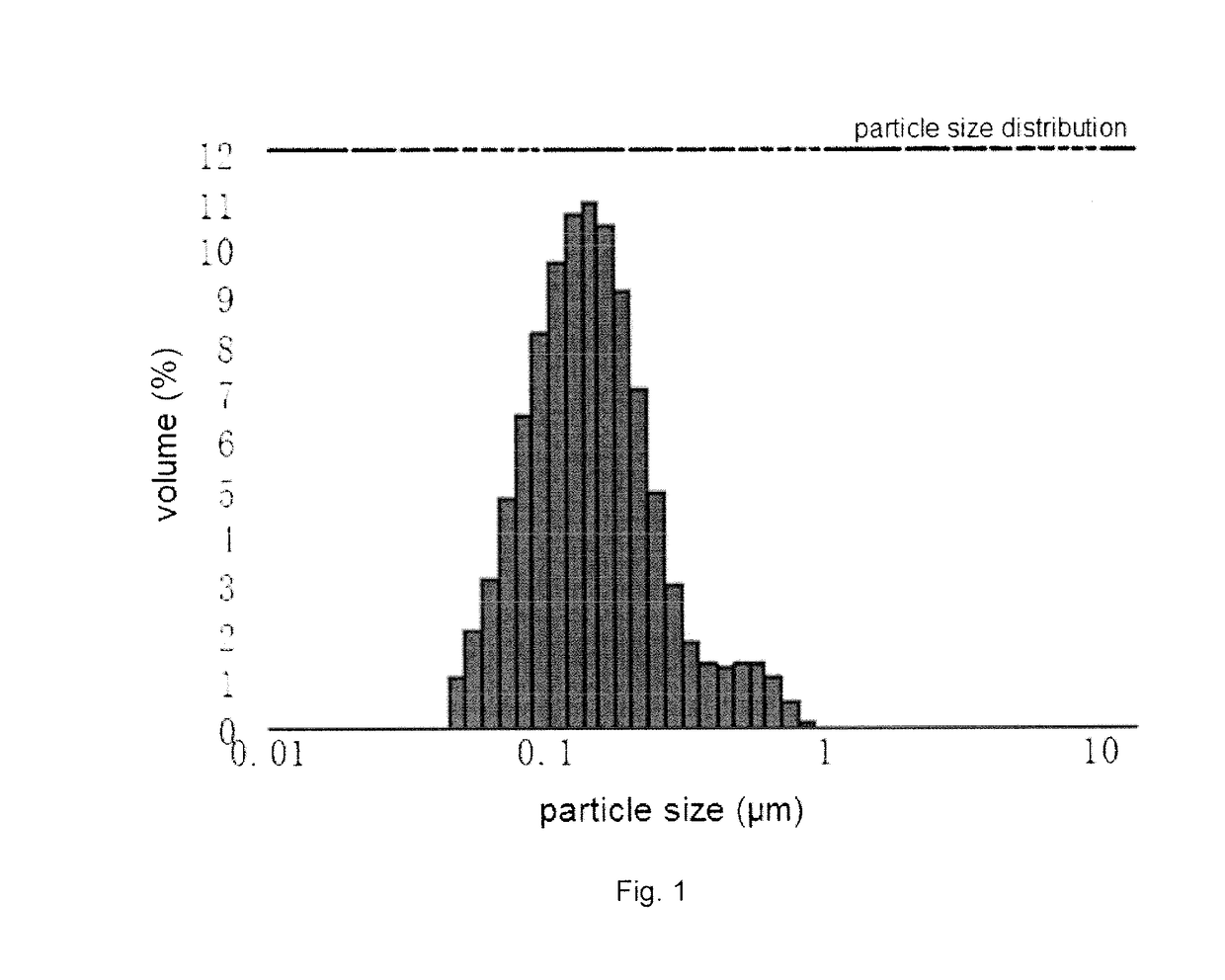
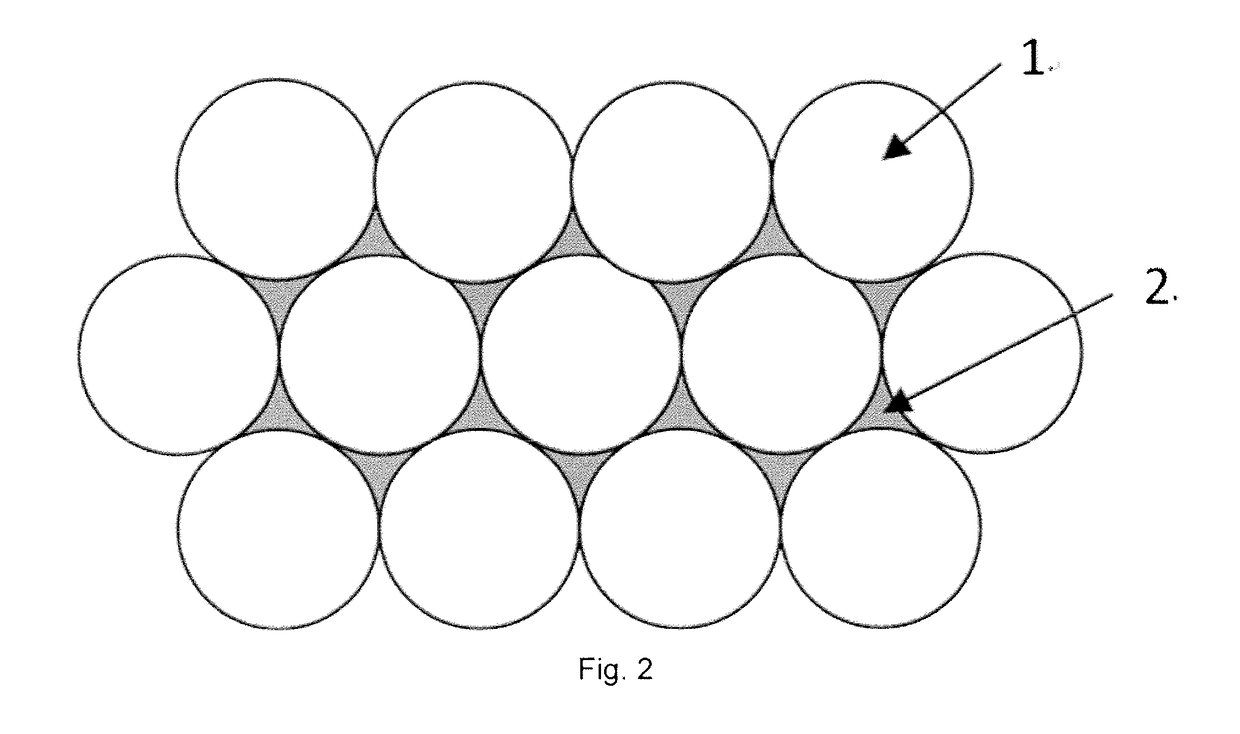
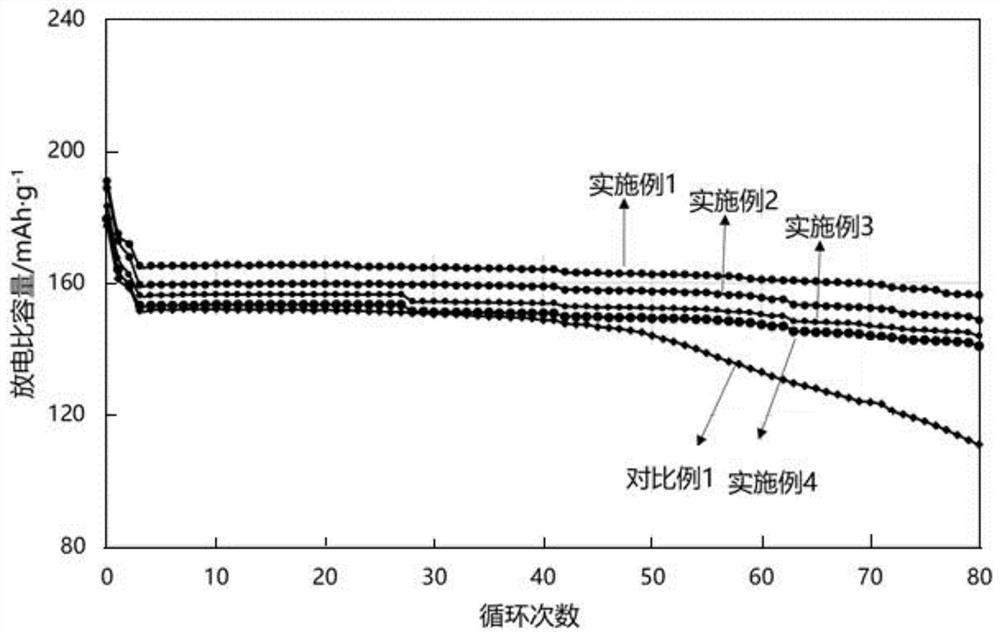
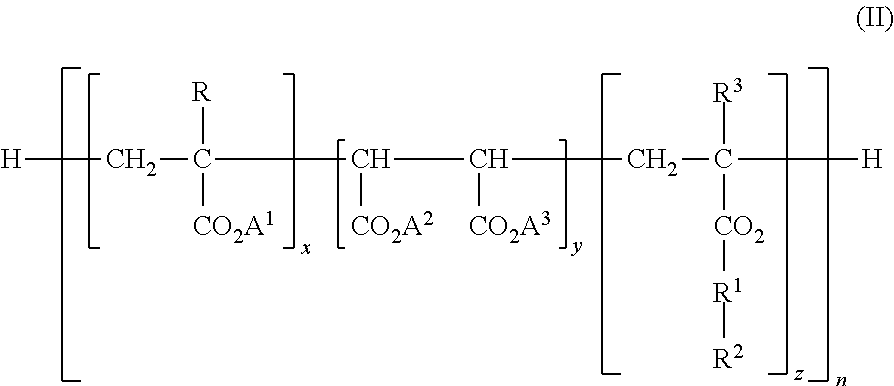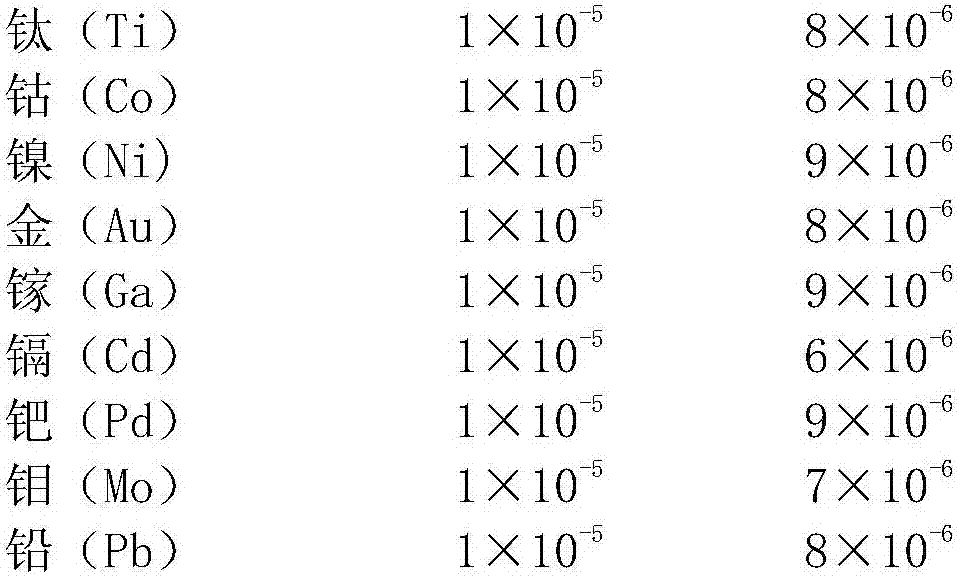Patents
Literature
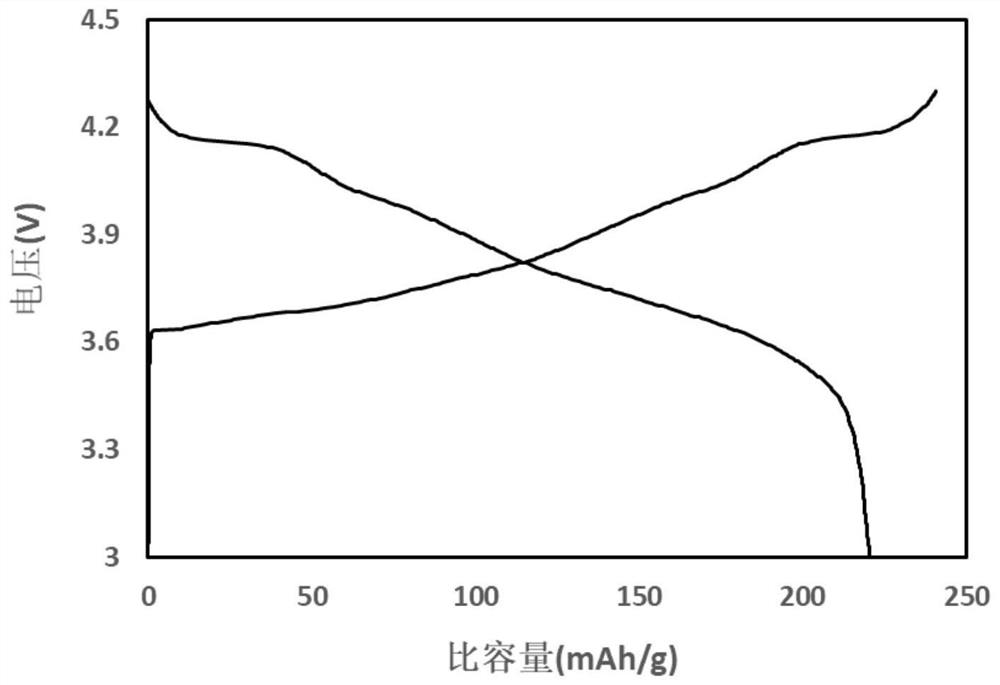
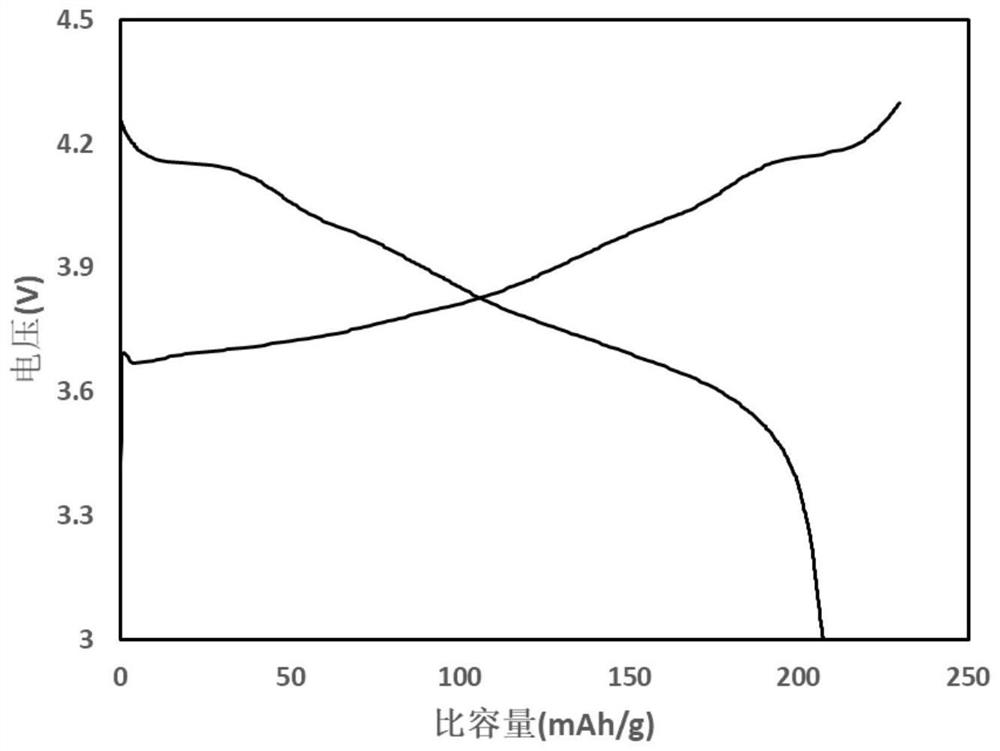
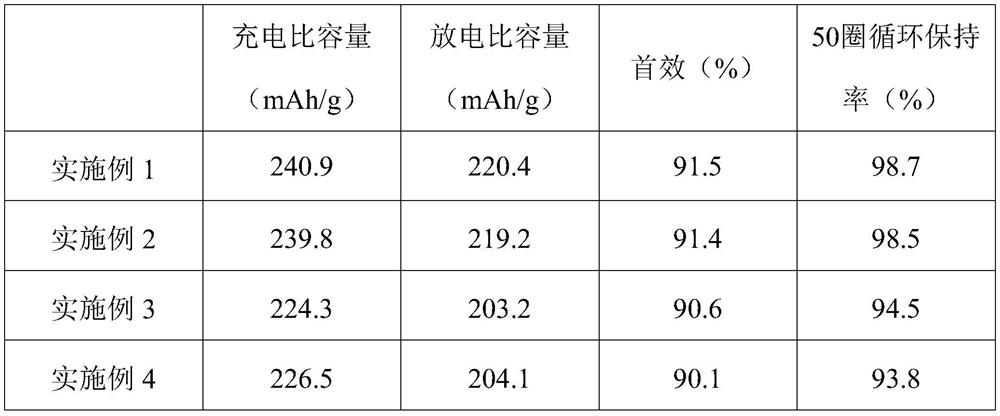
51results about "Boron oxides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
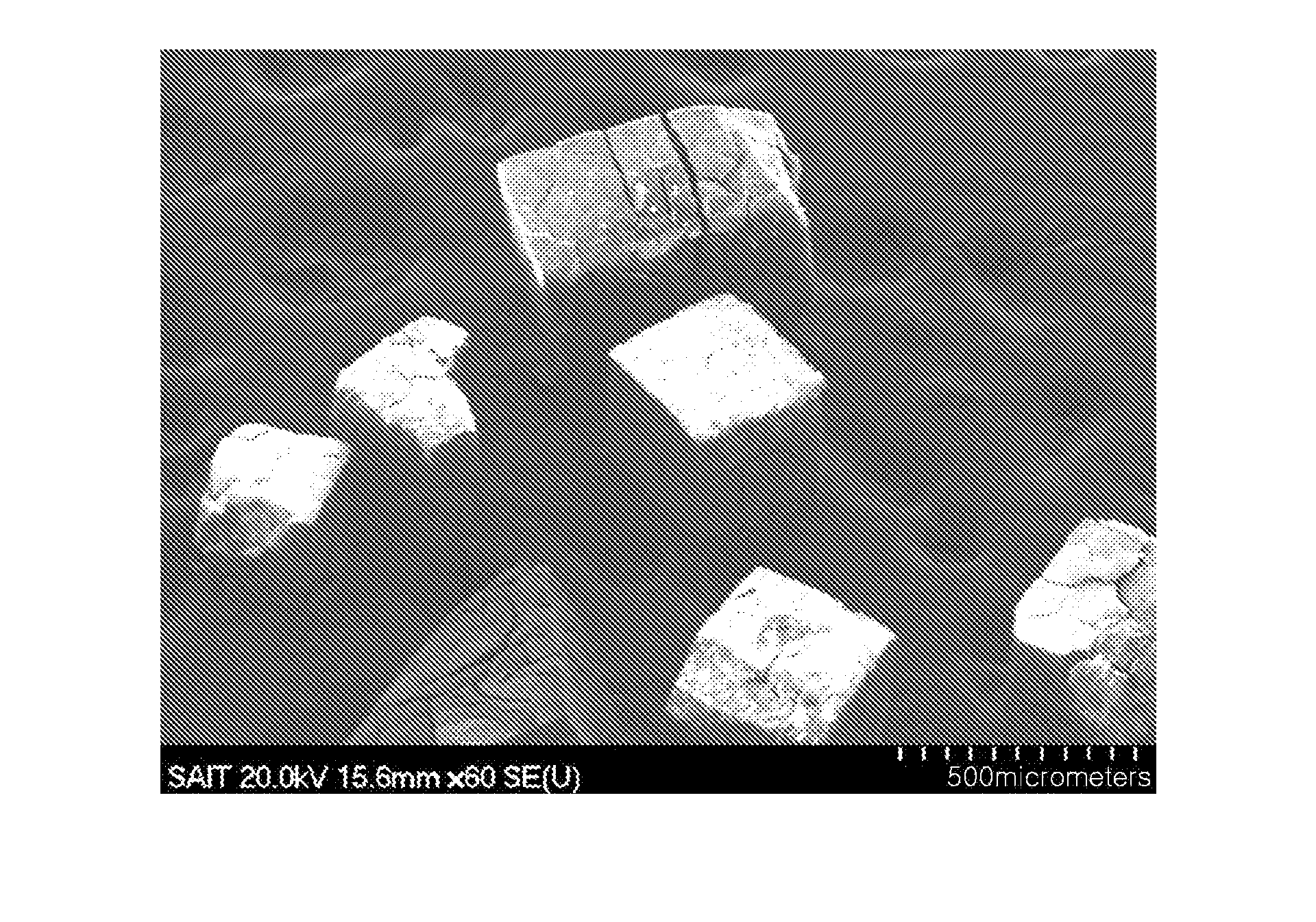
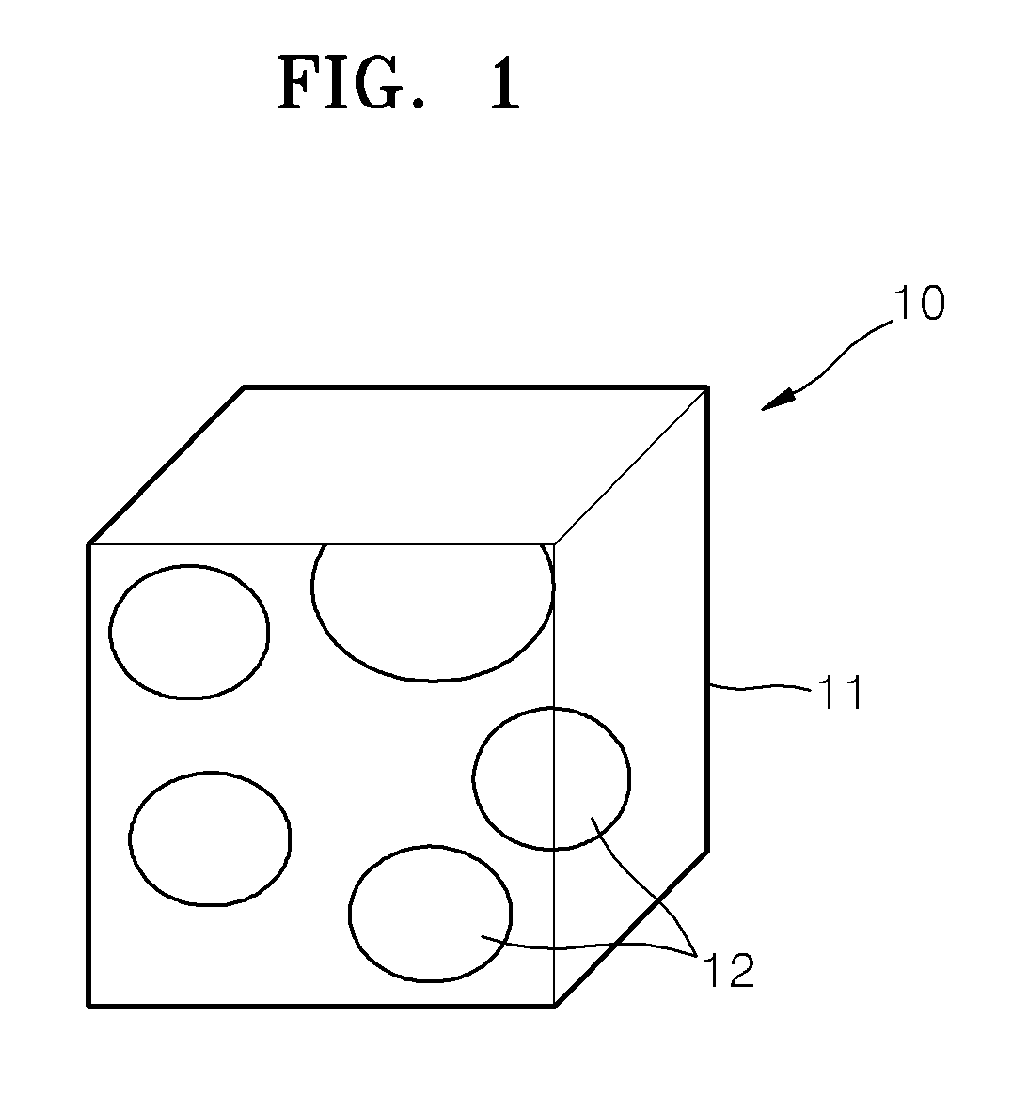
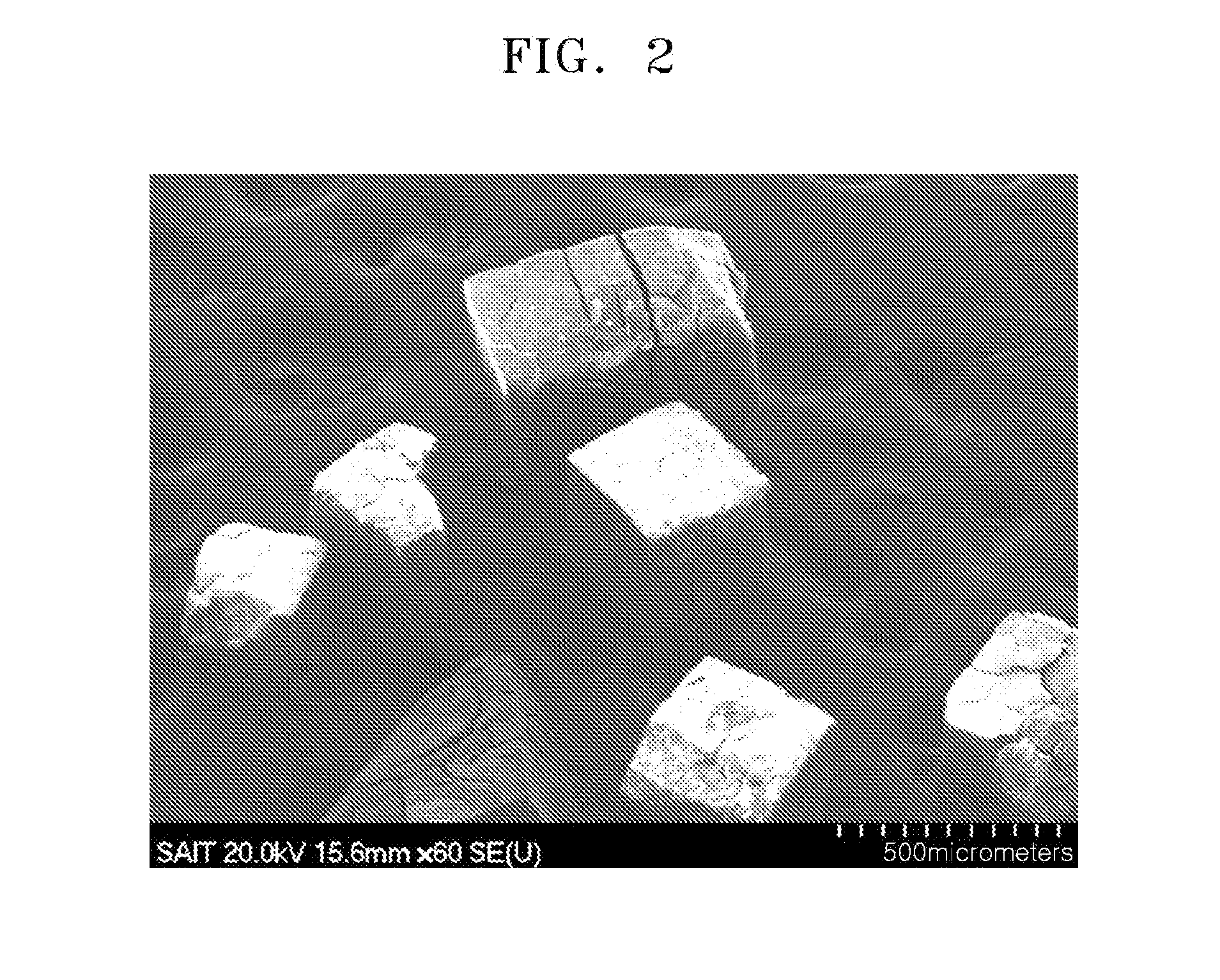
Hybrid porous material and methods of preparing the same
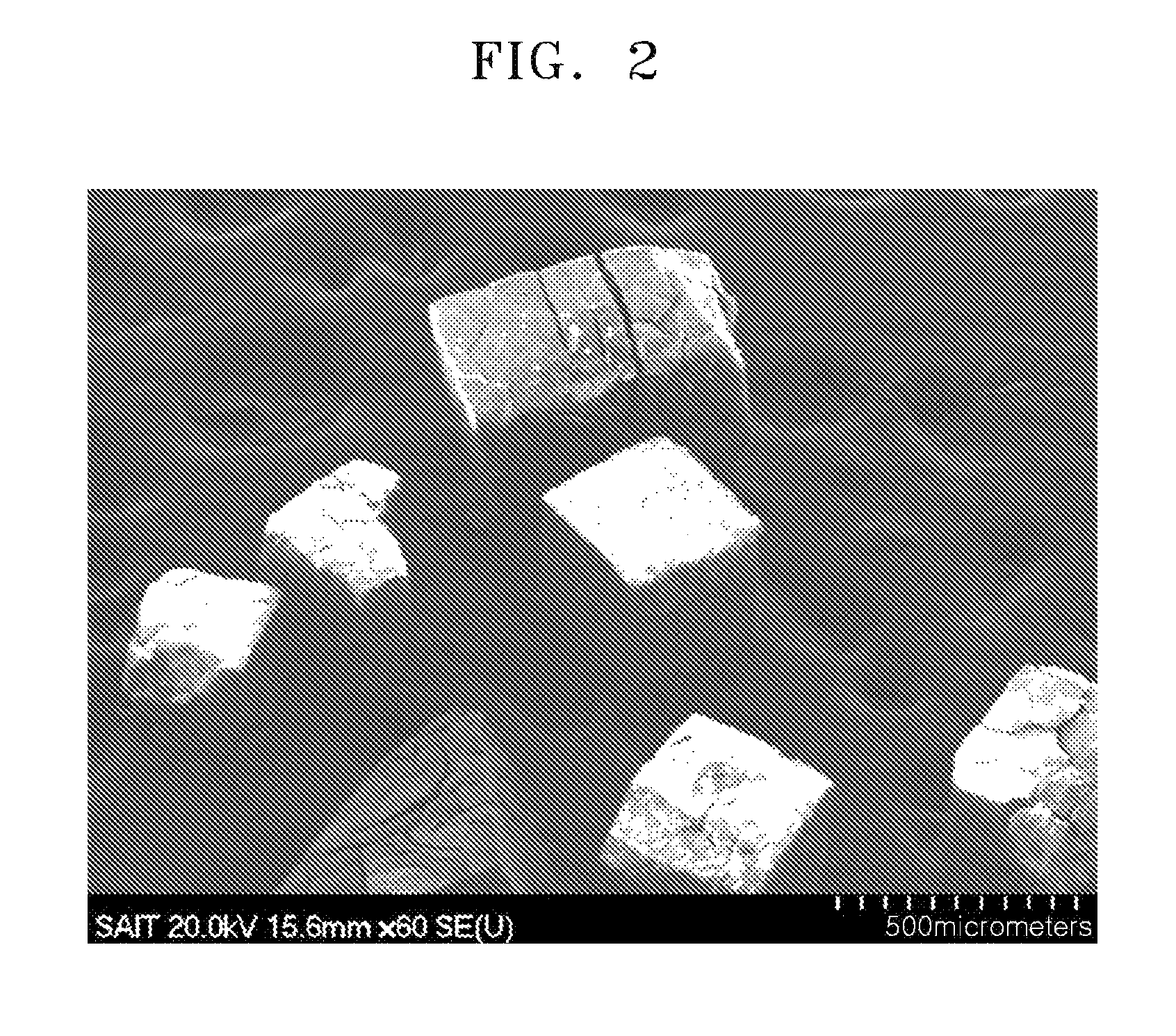
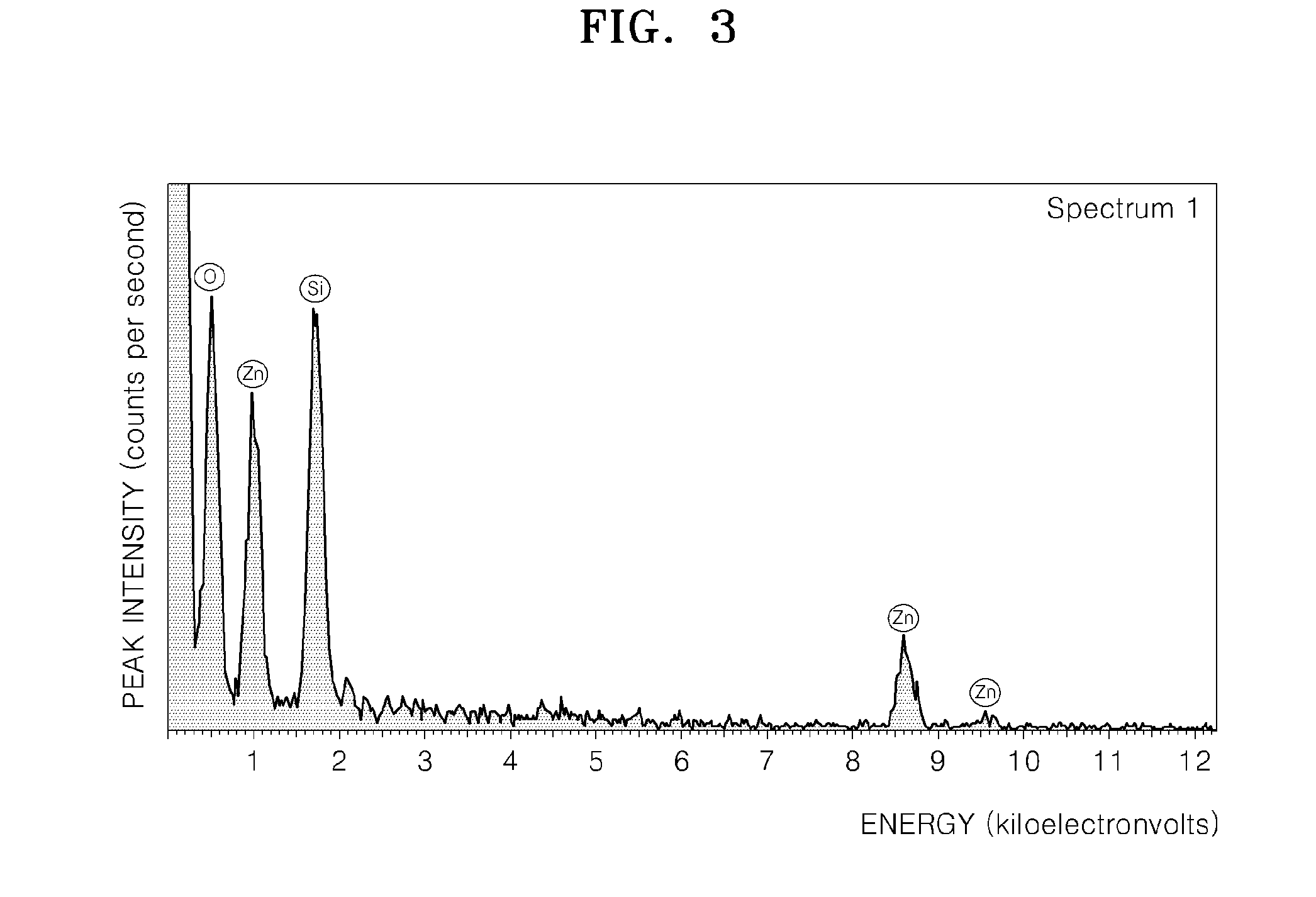
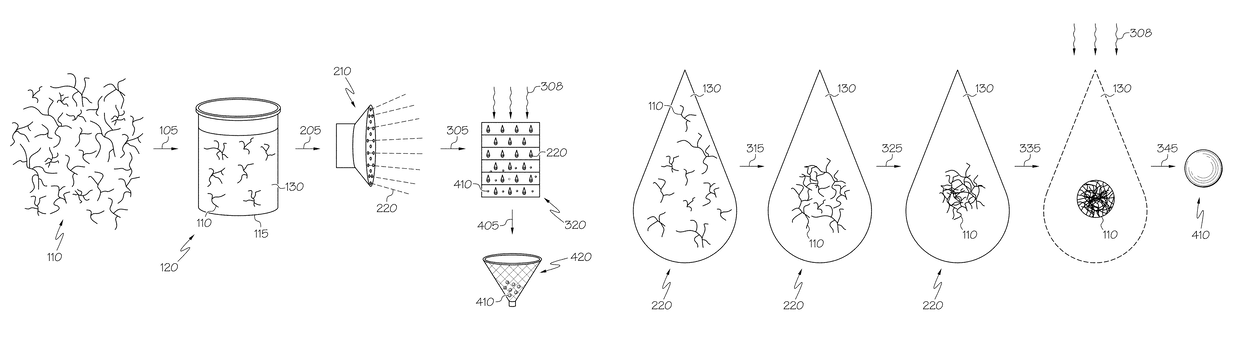
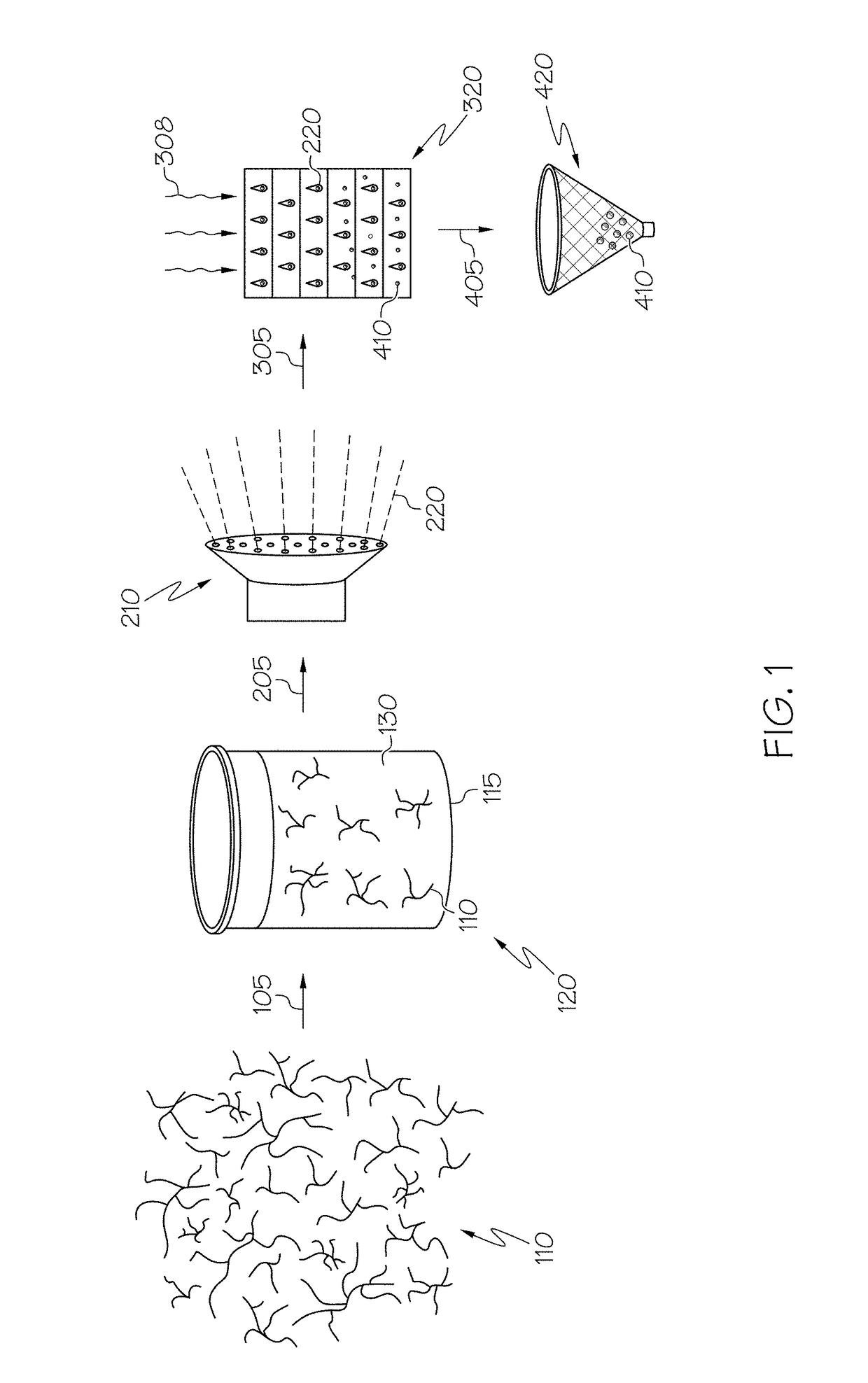
ActiveUS20110144365A1Silicon organic compoundsOxygen/ozone/oxide/hydroxideMaterials scienceChemical bond
A hybrid porous material including at least a first and a second porous material portion which are chemically bonded to each other and are each a different type of material.
Owner:SAMSUNG ELECTRONICS CO LTD
Electrolyte for power storage devices and nonaqueous electrolyte solution
ActiveCN111971842AGood initial propertiesExcellent cycle characteristicsHybrid capacitor electrolytesAlkali metal silicatesElectrolytic agentAcyl group
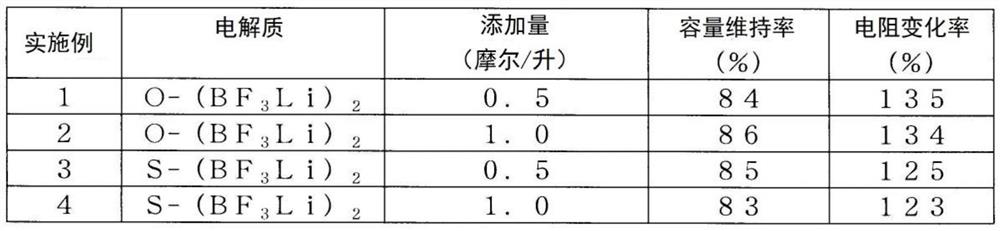
The purpose of the present invention is to provide: an enhanced electrolyte for power storage devices in which electrical resistance is reduced and it is possible to maintain high capacity even afterrepeated charging and discharging; and a power storage device. Provided is an electrolyte for power storage devices that is characterized by containing a lithium-containing complex compound represented by formula (1), formula (2), formula (3), formula (4), or formula (5) indicated below. Formula (1): (Li)m(A)n(UFx)y. Formula (2): (Li)m(Si)n(O)q(UFx)y (in formulas (1) and (2), A is O, S, P, or N, Uis a boron atom or a phosphorus atom, m and n each independently represent a number between 1 and 6 inclusive, q is a number between 1 and 12 inclusive, x is 3 or 5, and y is a number between 1 and 6inclusive). Formula (3): (Li)m(O)n(B)p(OWFq)x (in the formula, W is a boron atom or a phosphorus atom, m, p, and x each independently represent a number between 1 and 15 inclusive, n is a number between 0 and 15 inclusive, and q is 3 or 5). Formula (4): (Li)m(B)p(O)n(OR)y(OWFq)x (in the formula, W is a boron atom or a phosphorus atom, n is a number between 0 and 15 inclusive, p, m, x, and y eachindependently represent a number between 1 and 12 inclusive, q is 3 or 5, R is hydrogen, an alkyl group, an alkenyl group, an aryl group, a carbonyl group, a sulfonyl group, or a silyl group, and these groups may have a fluorine atom, an oxygen atom, and other substituent groups). Formula (5): (Li)m(O)n(B)p(OOC-(A)z-COO)y(OWFq)x (in the formula, W is a boron atom or a phosphorus atom, A is a grouphaving six carbon atoms and may be an alkylene group, an alkenylene group, an alkynylene group, a phenylene group, or an alkylene group having an oxygen atom or a sulfur atom in the main chain thereof, m, p, x, and y each independently represent a number between 1 and 20 inclusive, n is a number between 0 and 20 inclusive, n is a number between 0 and 15 inclusive, z is 0 or 1, and q is 3 or 5).
Owner:富山药品工业株式会社
Carbon combustion synthesis of oxides
ActiveUS20060097419A1Low production costWell mixedPigmenting treatmentAlkaline earth titanatesOxide ceramicSelf-propagating high-temperature synthesis
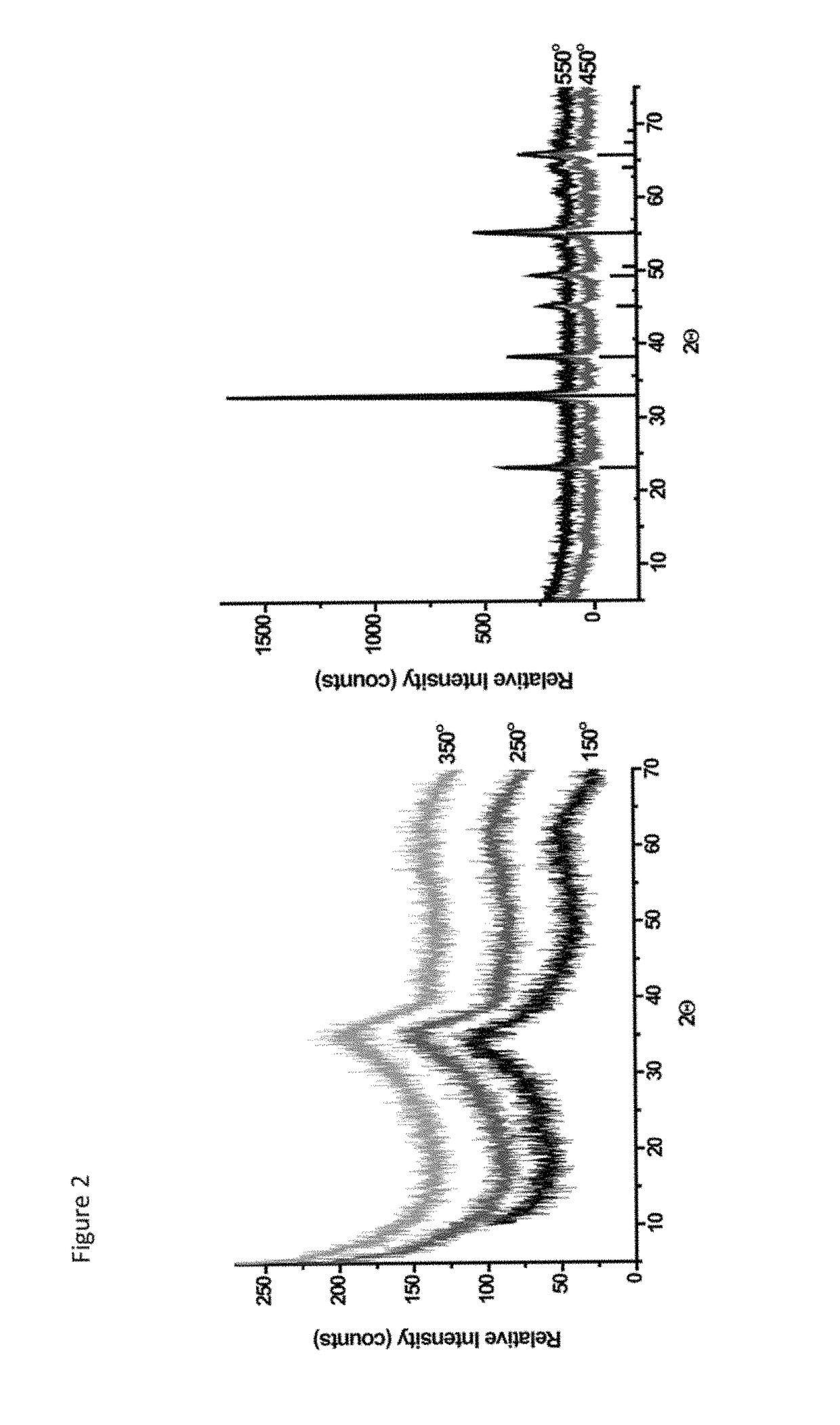
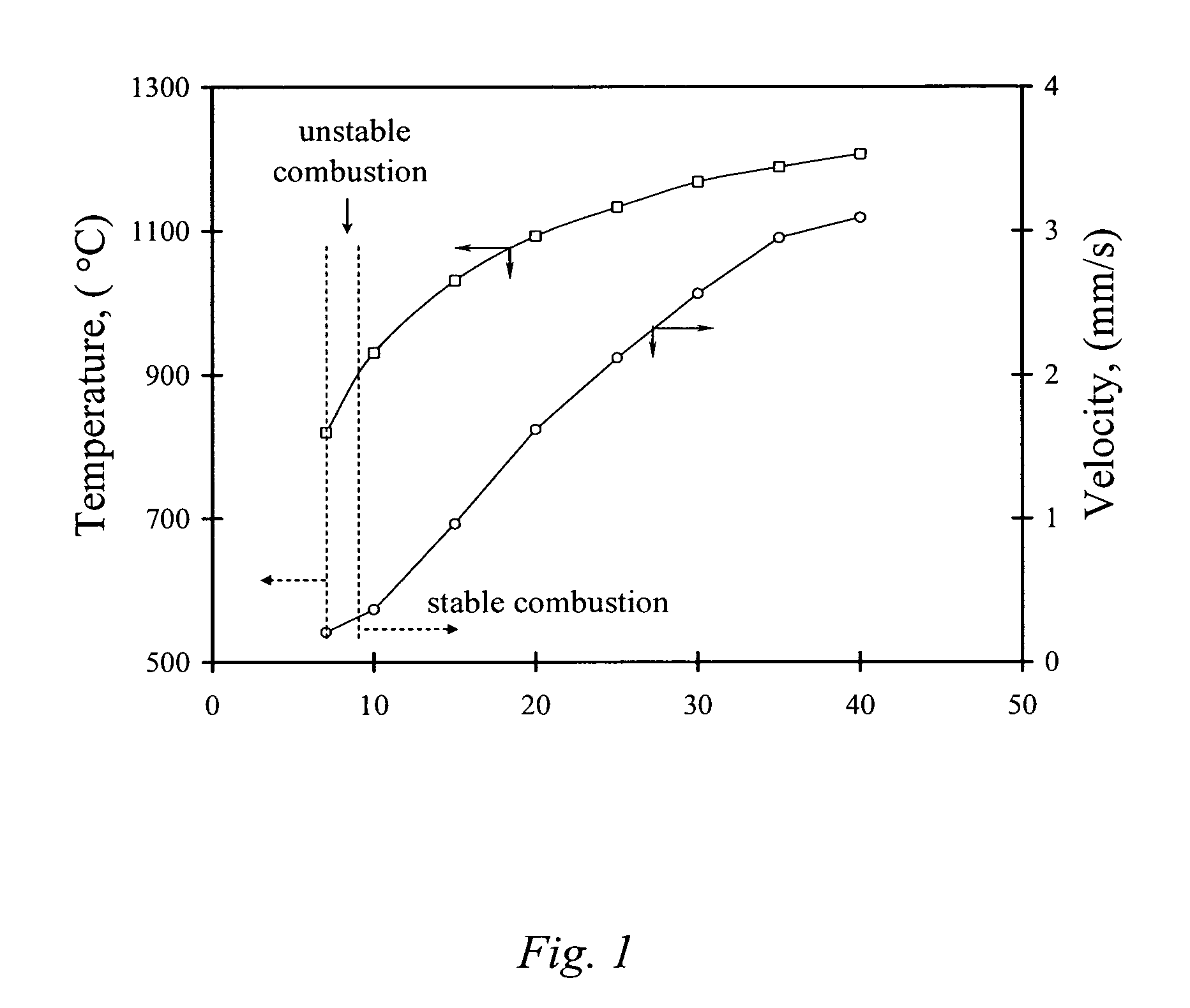
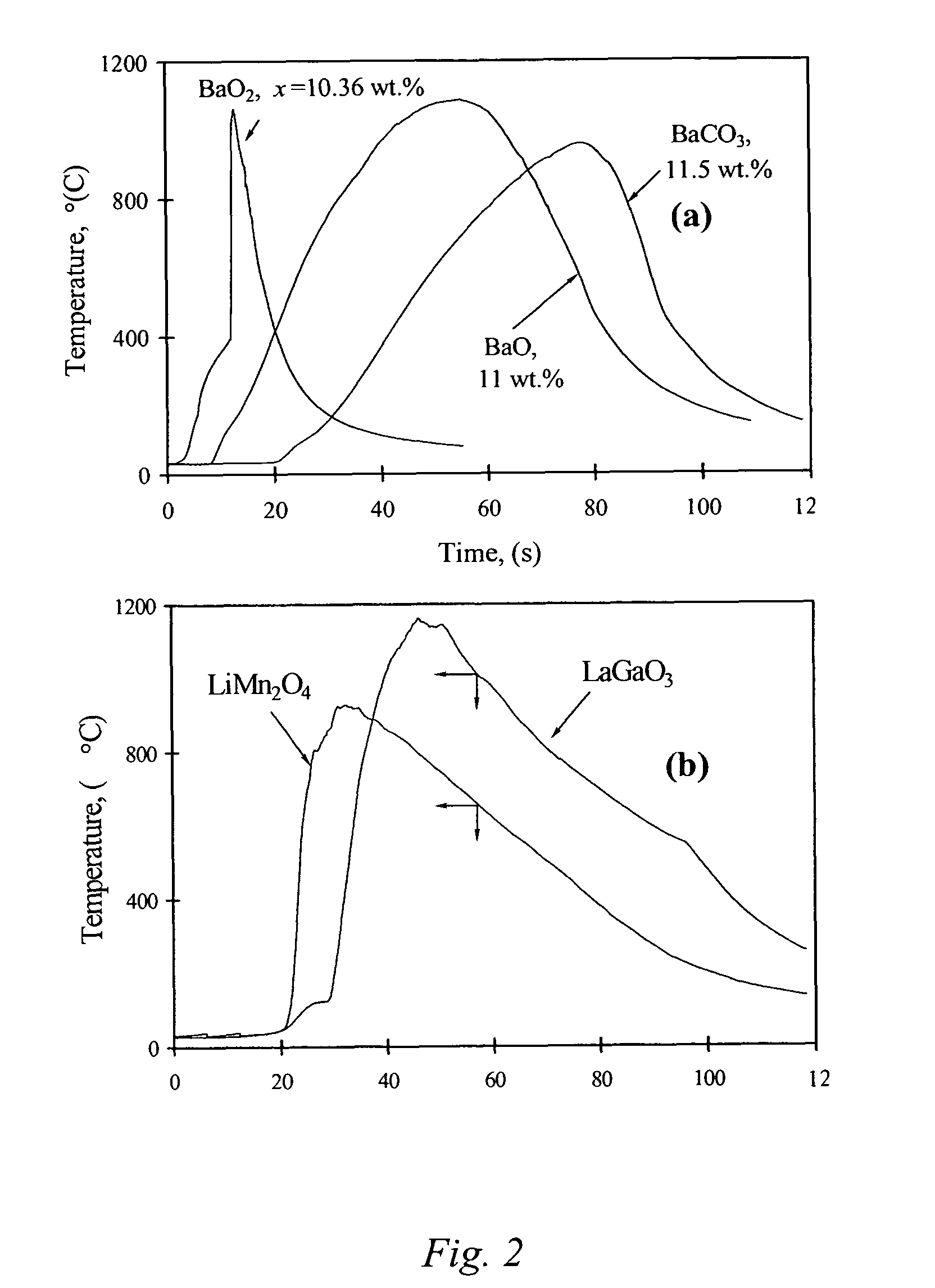
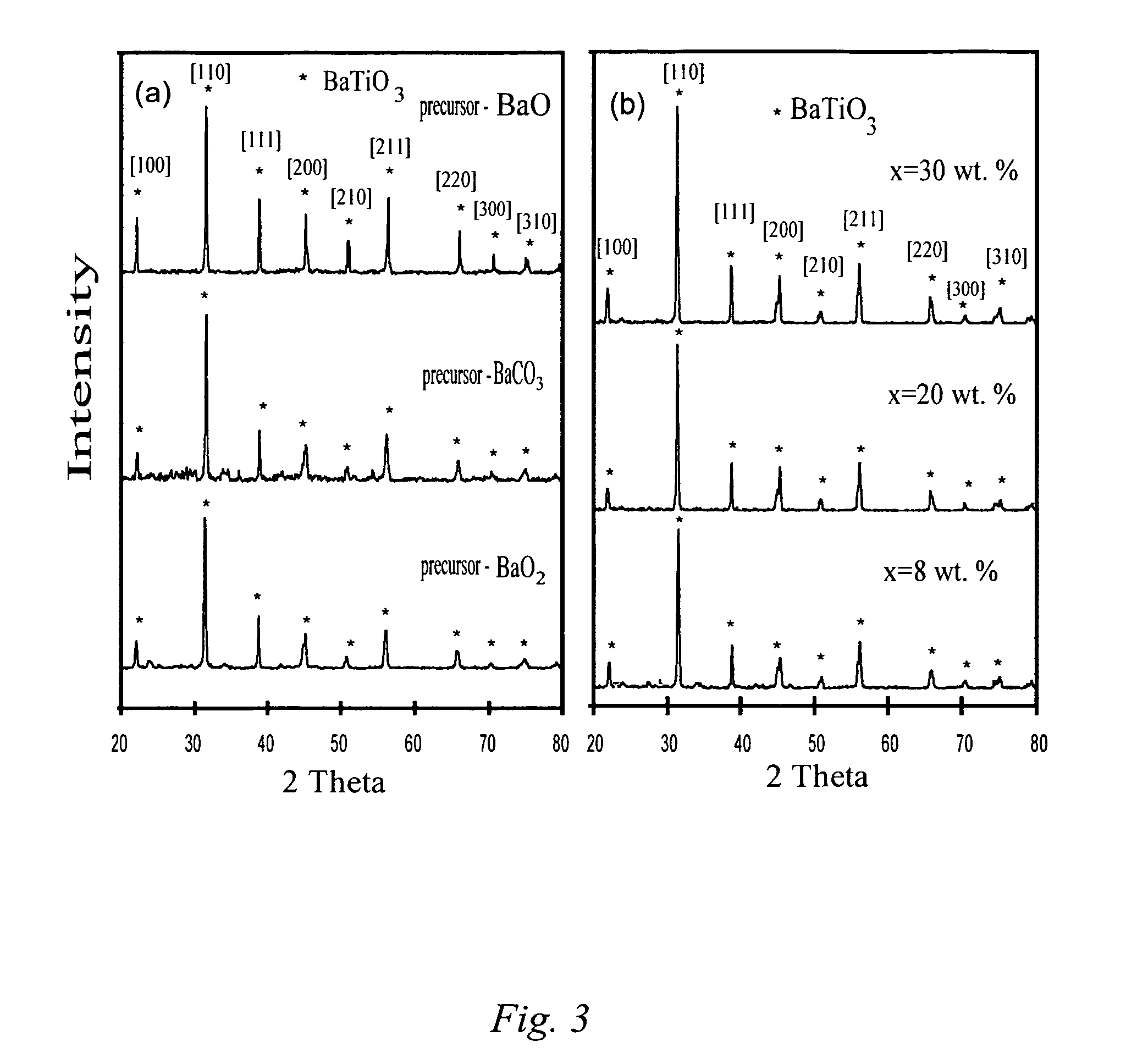
The present invention is generally directed to a novel, economic synthesis of oxide ceramic composites. Methods of the present invention, referred to as carbon combustion synthesis of oxides (CCSO), are a modification of self-propagating high-temperature synthesis (SHS) methods in which the heat needed for the synthesis is generated by combustion of carbon in oxygen rather than that of a pure metal. This enables a more economic production of the ceramic material and minimizes the presence of intermediate metal oxides in the product. The reactant mixture generally comprises at least one oxide precursor (e.g., a metal or non metal oxide, or super oxide, or nitride, or carbonate, or chloride, or oxalate, or halides) as a reactant, but no pure metal. Pure carbon in the form of graphite or soot is added to the reactant mixture to generate the desired heat (upon ignition). The mixture is placed in a reactor and exposed to gaseous oxygen. The high-temperature exothermic reaction between the carbon and oxygen generates a self-sustaining reaction in the form of a propagating temperature wave that causes a reaction among the reactants. The reaction proceeds rapidly following ignition, and the final product comprises simple and / or complex oxides of elements present in the oxide precursor(s). CCSO also enables synthesis of oxides that cannot be produced by conventional SHS, such as when the pure metal is pyrophoric (such as Li or La) or such as when it melts at room temperature (e.g., Ga) or such as the combustion heat of the metal is relatively low.
Owner:UNIV HOUSTON SYST
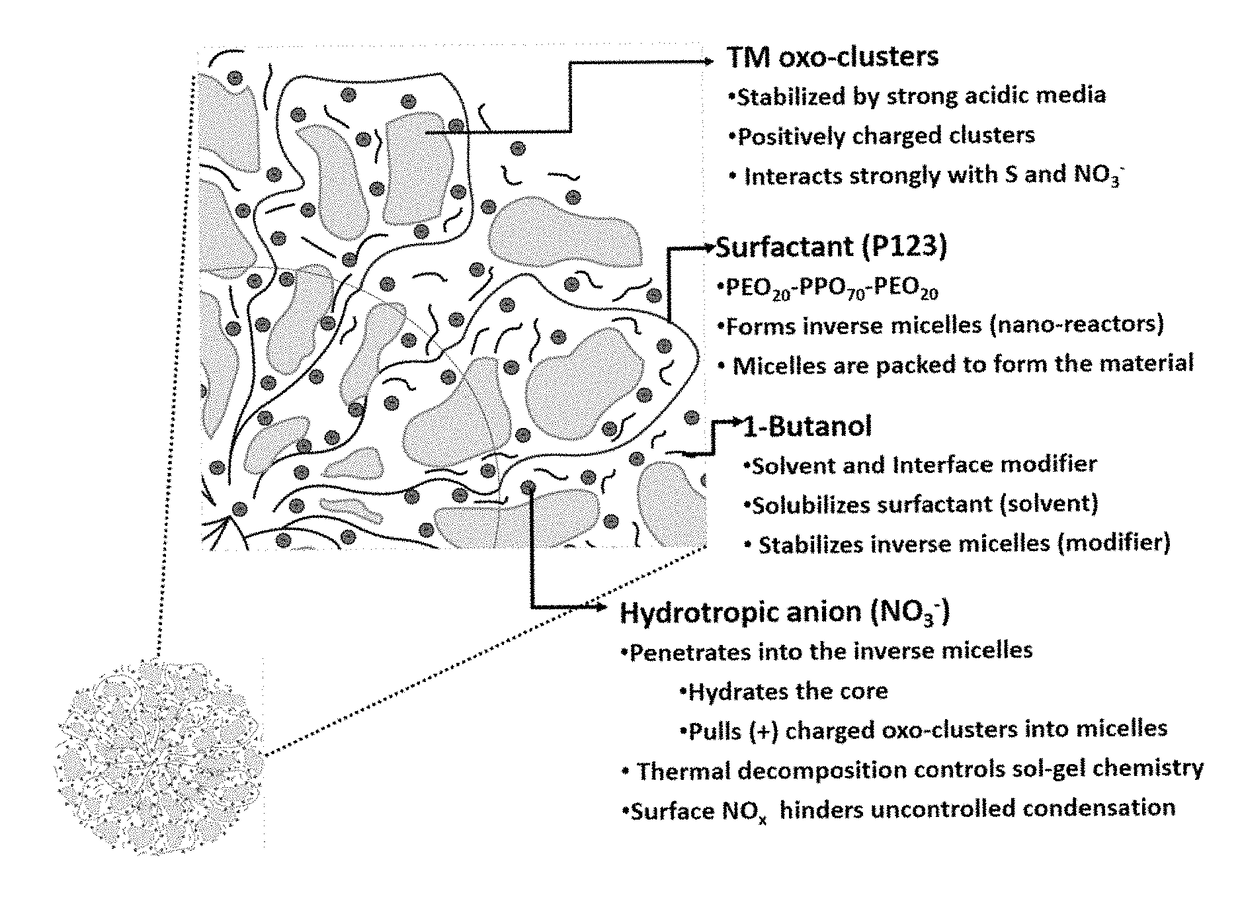
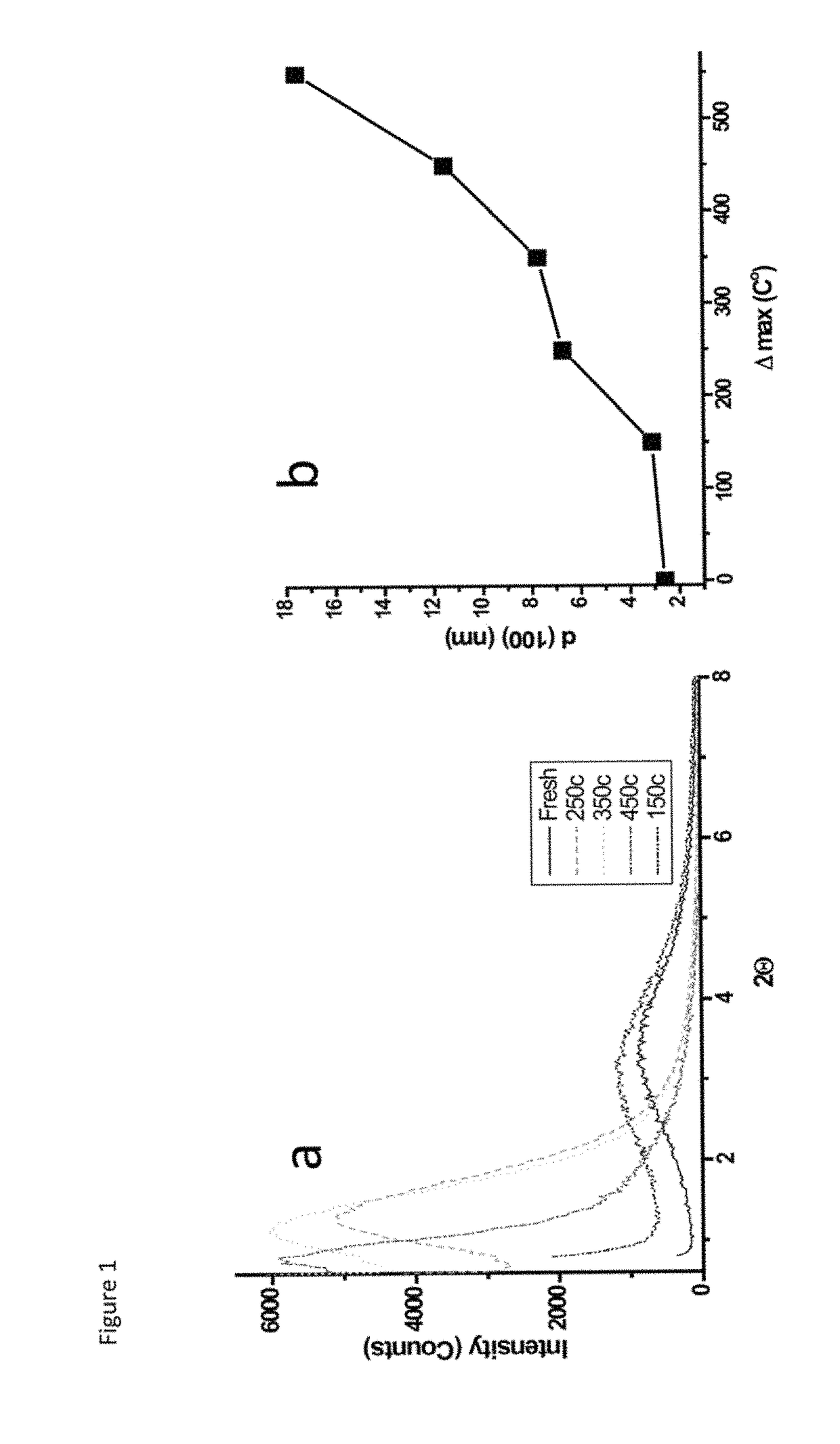
Mesoporous metal oxides and processes for preparation thereof
ActiveUS9452933B2Simple heat treatment processDifferent porous structureMaterial nanotechnologySilicaLanthanideCrystallinity
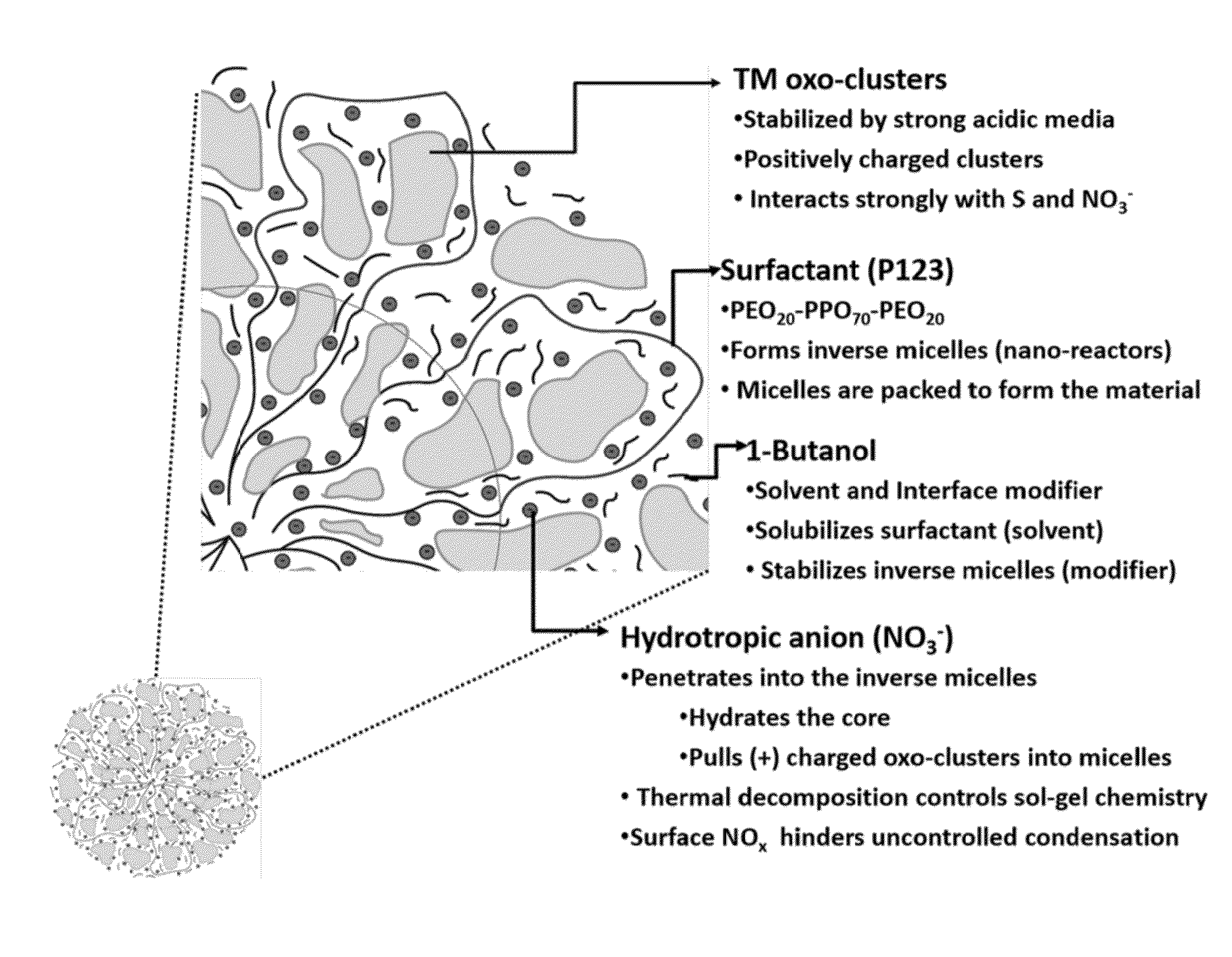
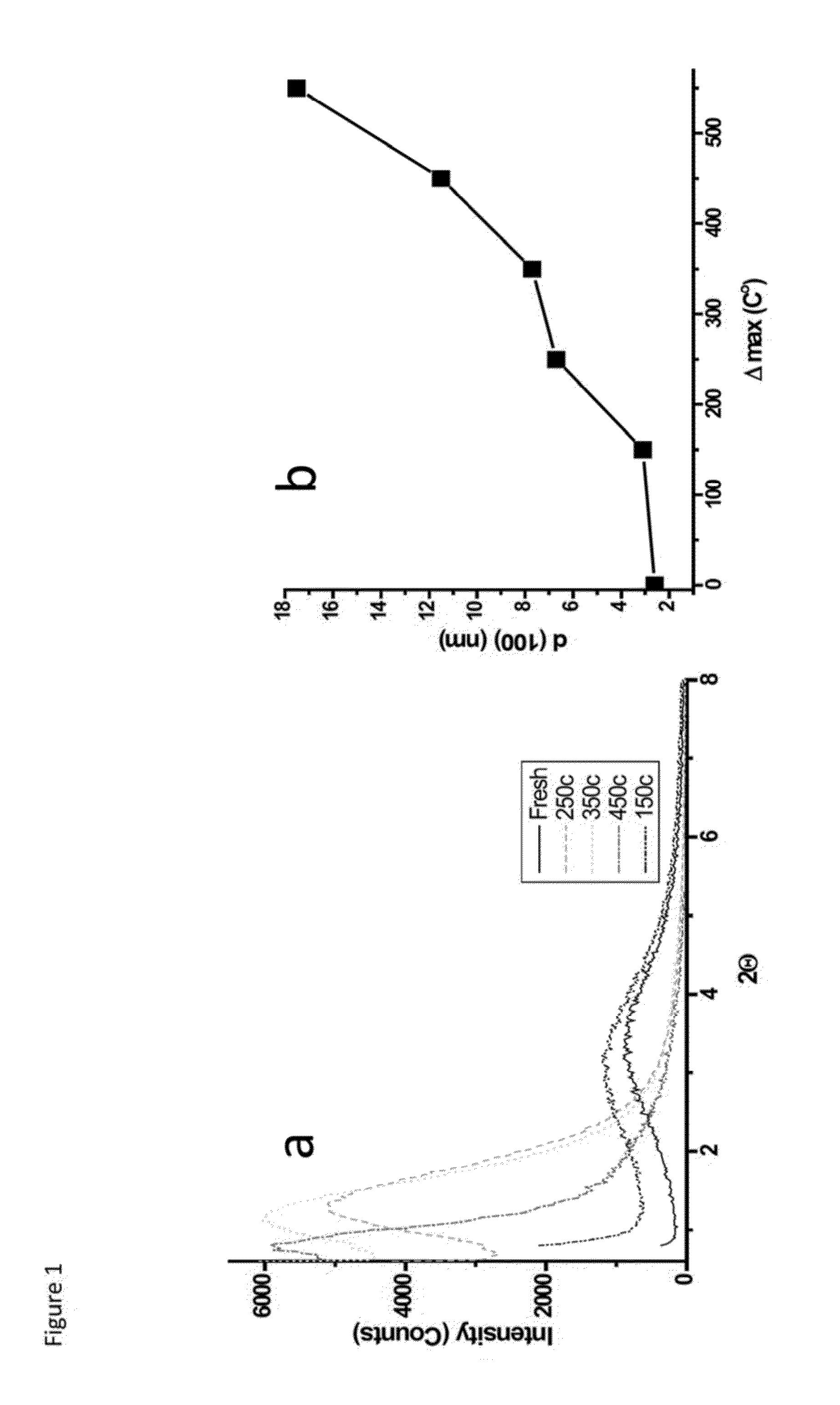
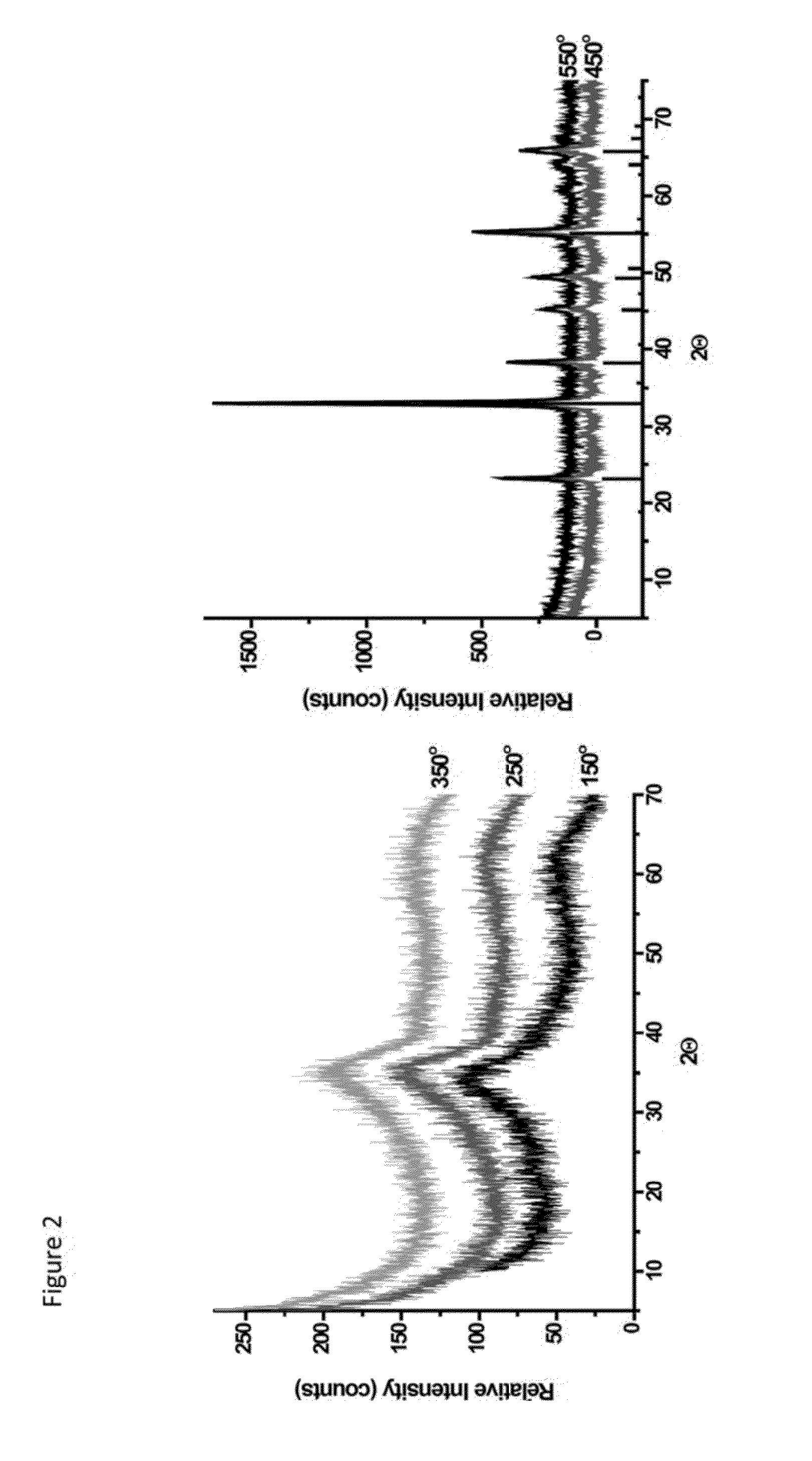
A process for preparing a mesoporous metal oxide, i.e., transition metal oxide, Lanthanide metal oxide, a post-transition metal oxide and metalloid oxide. The process comprises providing a micellar solution comprising a metal precursor, an interface modifier, a hydrotropic ion precursor, and a surfactant; and heating the micellar solution at a temperature and for a period of time sufficient to form the mesoporous metal oxide. A mesoporous metal oxide prepared by the above process. A method of controlling nano-sized wall crystallinity and mesoporosity in mesoporous metal oxides. The method comprises providing a micellar solution comprising a metal precursor, an interface modifier, a hydrotropic ion precursor, and a surfactant; and heating the micellar solution at a temperature and for a period of time sufficient to control nano-sized wall crystallinity and mesoporosity in the mesoporous metal oxides. Mesoporous metal oxides and a method of tuning structural properties of mesoporous metal oxides.
Owner:UNIV OF CONNECTICUT
Hybrid porous material and methods of preparing the same
ActiveUS8552189B2Silicon organic compoundsOxygen/ozone/oxide/hydroxideChemical LinkageMaterials science
Owner:SAMSUNG ELECTRONICS CO LTD
Methods for processing fumed metallic oxides
ActiveUS9969621B2Large specific surface areaReduce risk of inhalationSilicaGranulation by liquid drop formationSuspended particlesGas phase
Novel methods for processing fumed metallic oxides into globular metallic oxide agglomerates are provided. The methodology may allow for fumed metallic oxide particles, such as fumed silica and fumed alumina particles, to be processed into a globular morphology to improve handling while retaining a desirable surface area. The processes may include providing fumed metallic oxide particles, combining the particles with a liquid carrier to form a suspension, atomizing the solution of suspended particles, and subjecting the atomized droplets to a temperature range sufficient to remove the liquid carrier from the droplets, to produce metallic oxide-containing agglomerations.
Owner:SAUDI ARABIAN OIL CO
Boron Compound Suspension
ActiveUS20130230605A1Avoid cloggingImpaired movementBiocideCosmetic preparationsHigh concentrationBoron containing
A suspension of a boron containing compound in the form of crystals, powder or granulate in a solvent which contain a carbomer as dispersant. This suspension is very stable, even at high concentrations, and exhibits favourable non-Newtonian viscosity behavior, which makes it suitable in a number of applications, such as for the control of fission reactions with the generation of electric power from nuclear energy.
Owner:C IP SA
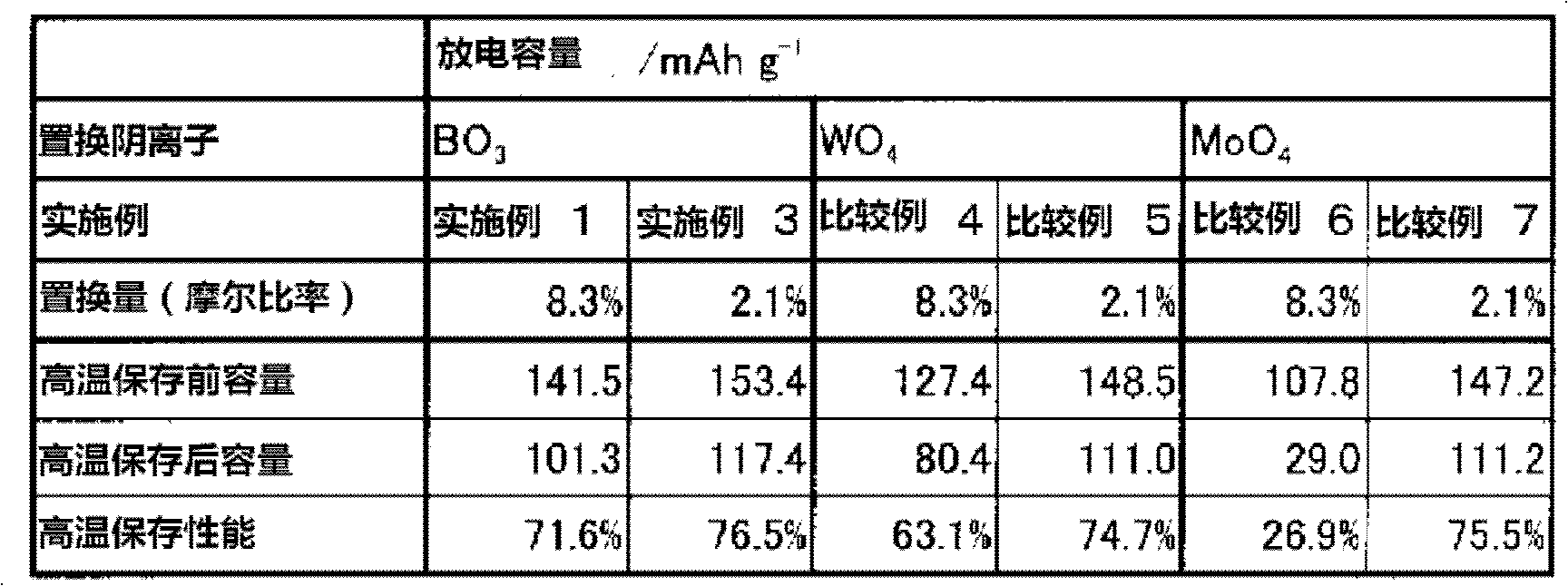
Positive active material for lithium secondary battery, electrode for lithium secondary battery, and lithium secondary battery
InactiveUS20120219862A1Easy to storeImprove high temperature storage performanceNon-metal conductorsConductive materialLithiumHigh temperature storage
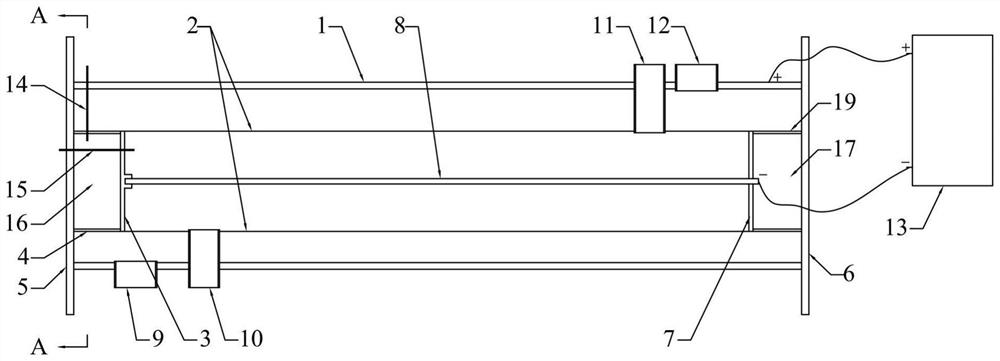
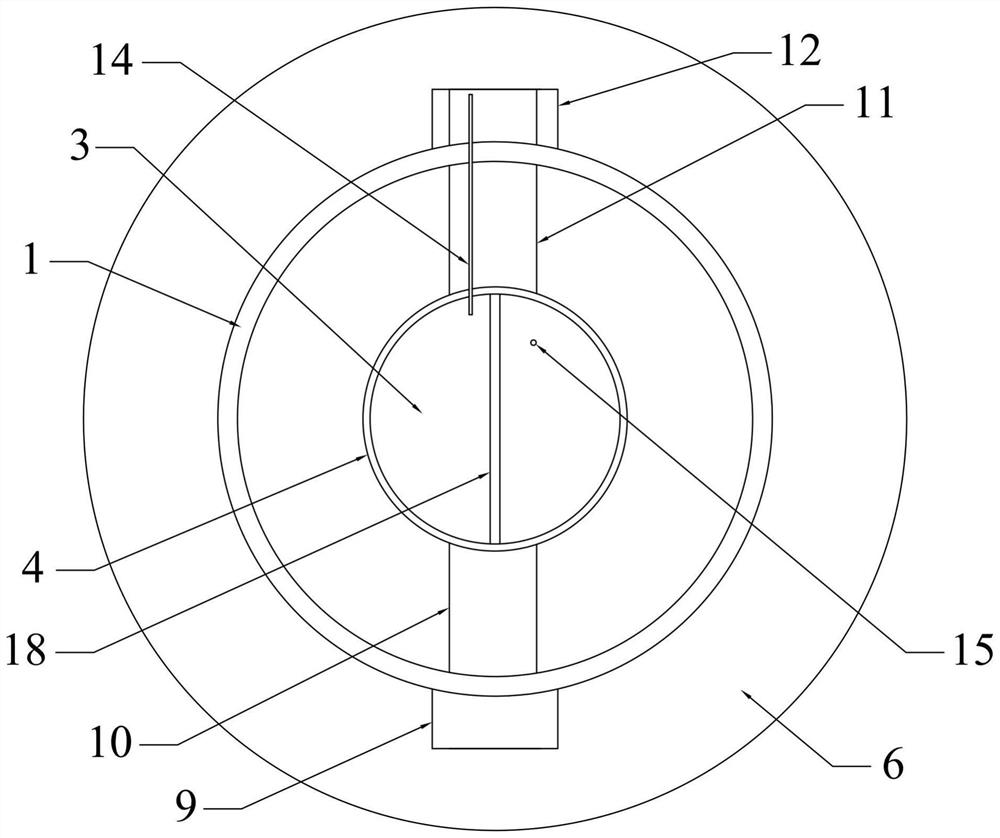
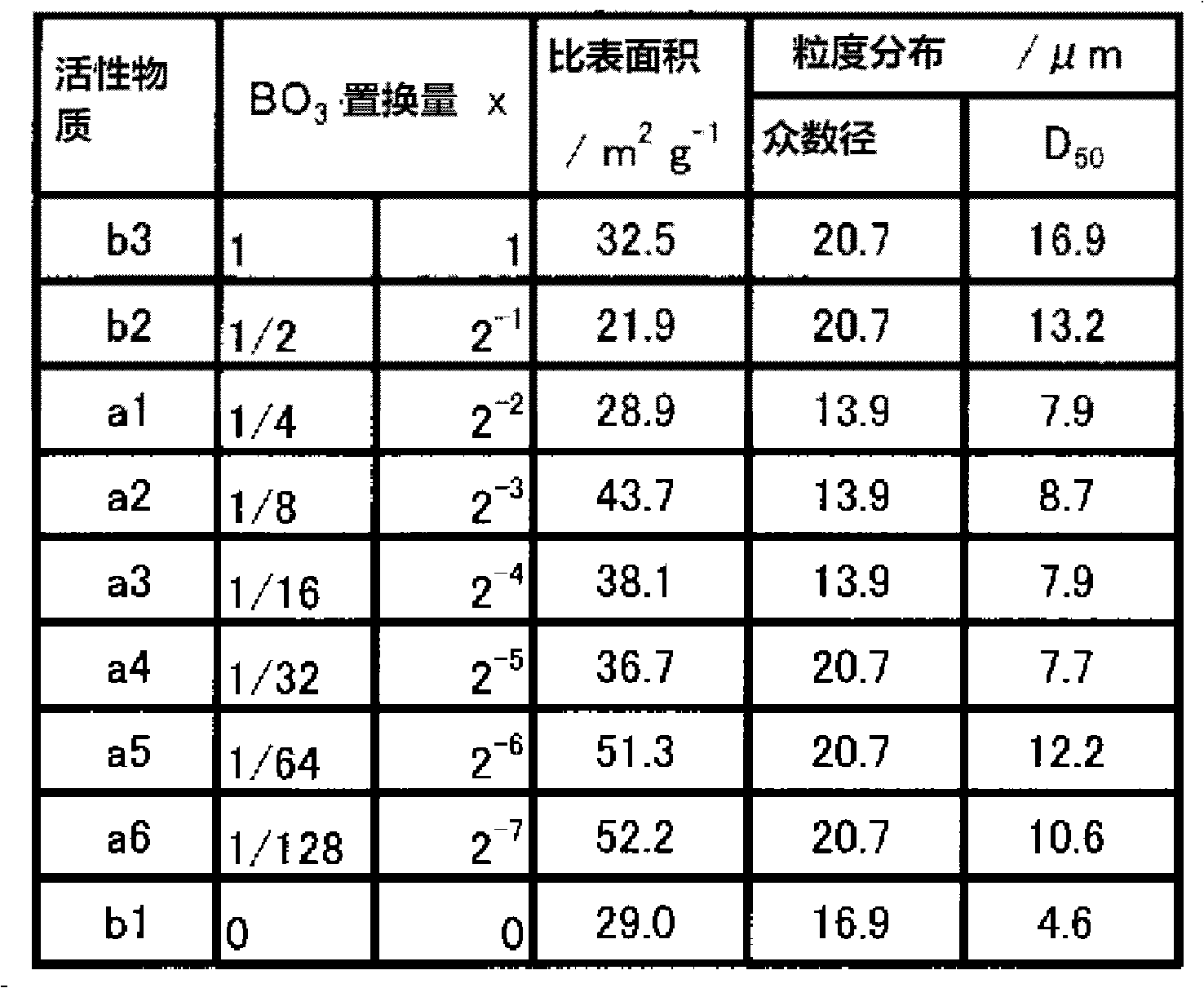
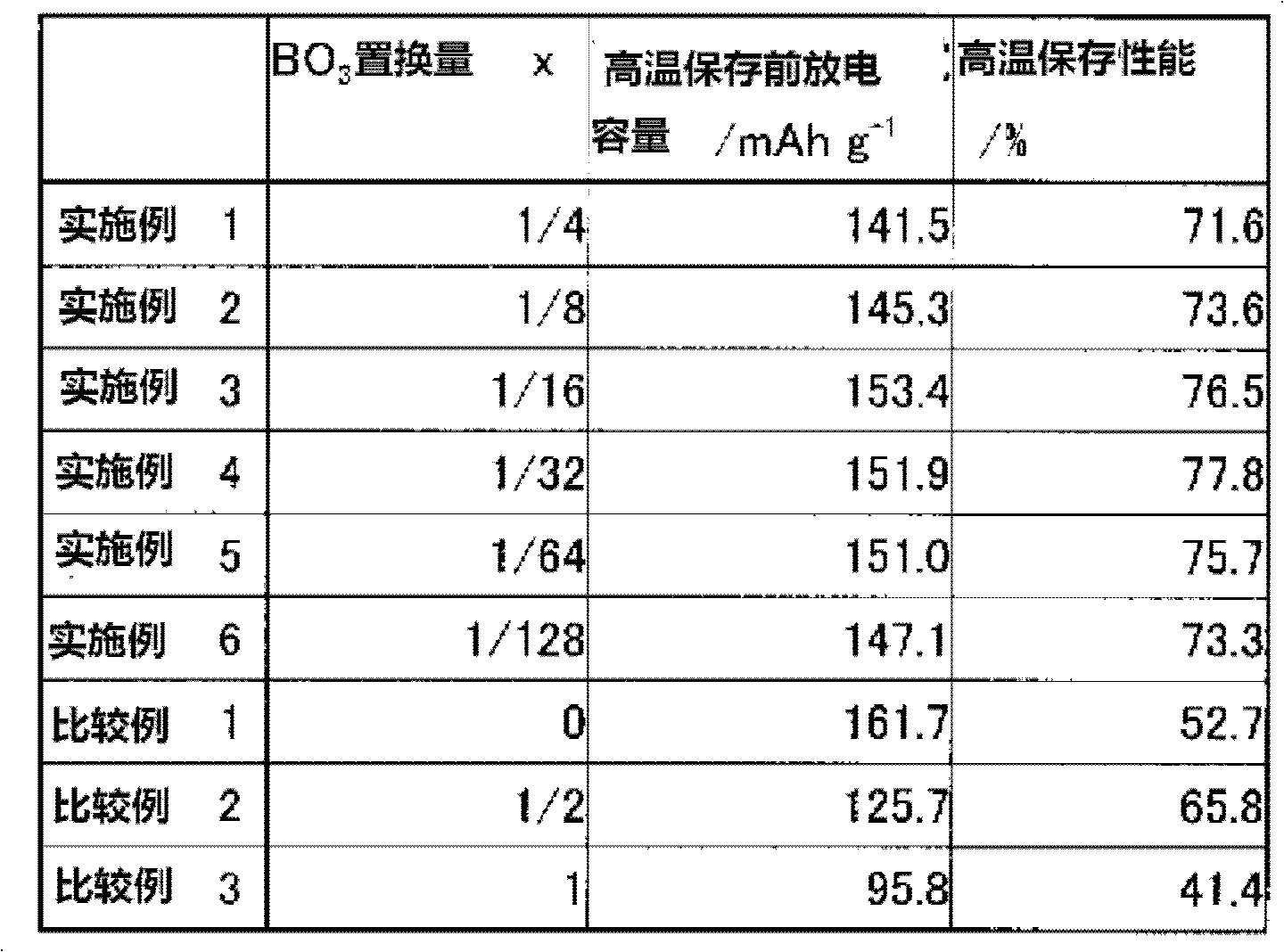
The present invention provides a Li3V2(PO4)3-based positive active material for a lithium secondary battery, which has high discharge capacity and excellent storage performance, particularly high-temperature storage performance; and a lithium secondary battery made using the positive active material. The positive active material for a lithium secondary battery has general formula Li3V2(PO4)3-x(BO3)x (0<x≦2−2). It is preferable that x be 2−7≦x≦2−3. Also provided are a positive electrode for a lithium secondary battery containing the positive active material; and a lithium secondary battery including the positive electrode.
Owner:GS YUASA INT LTD
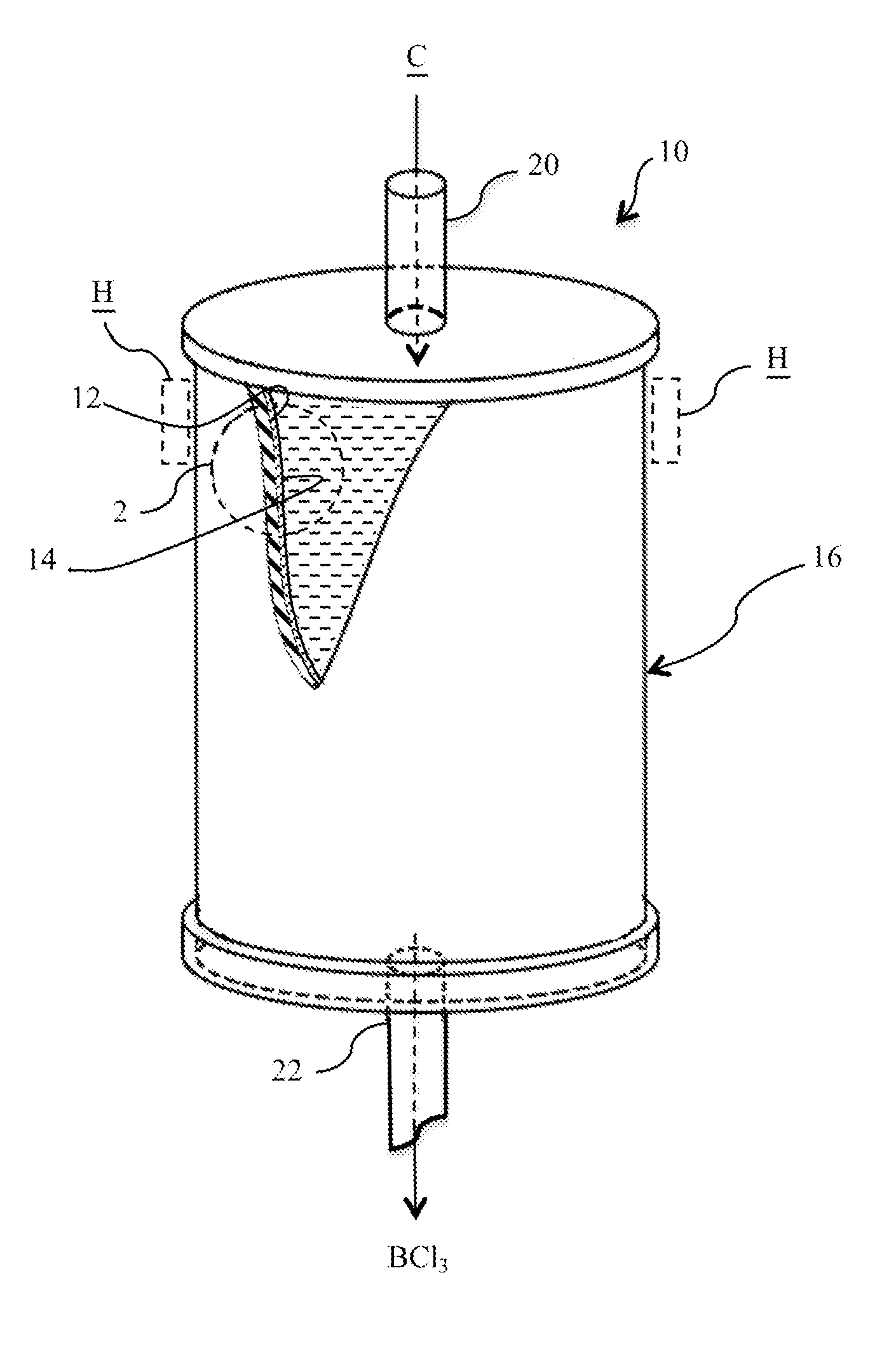
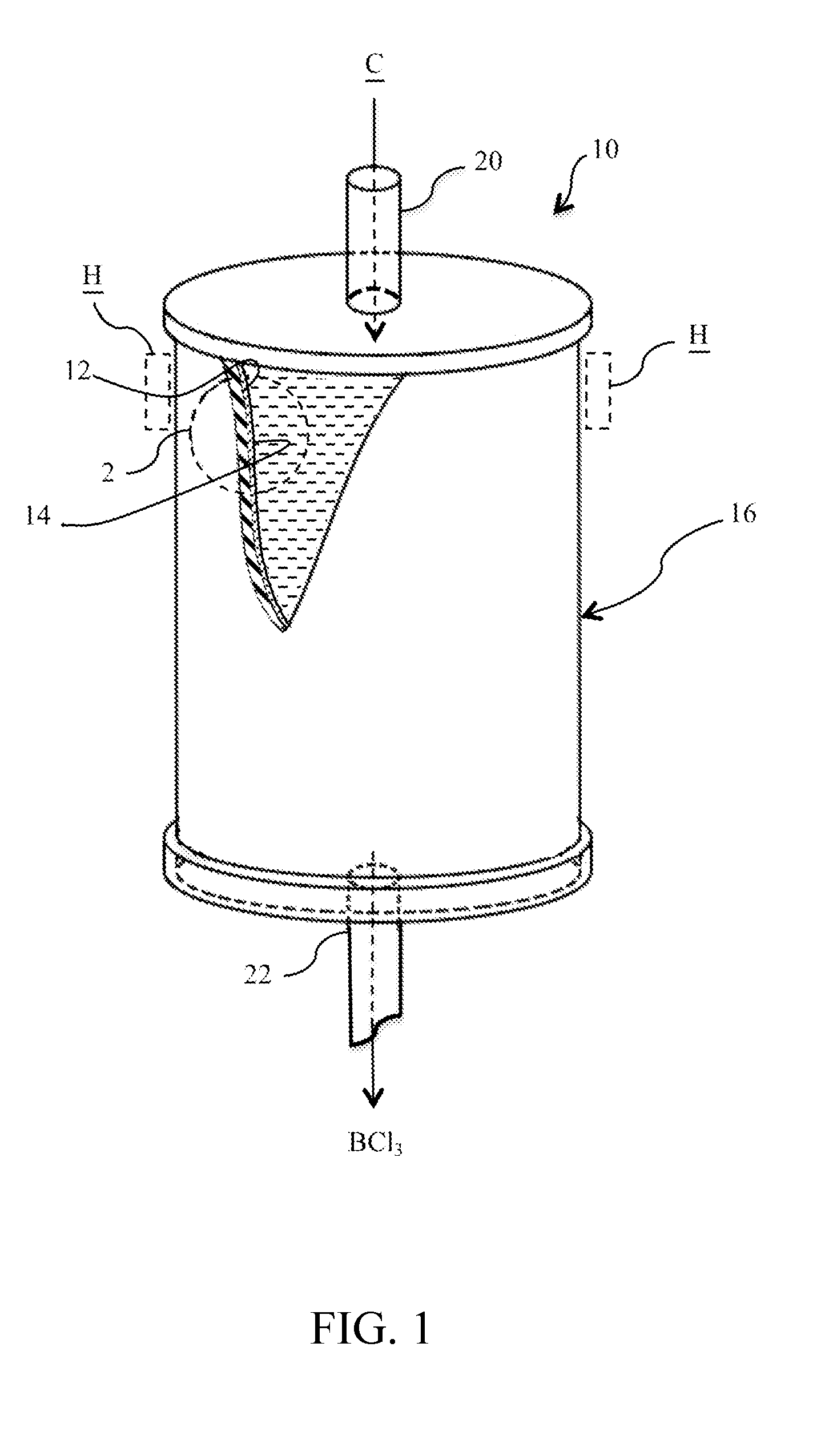
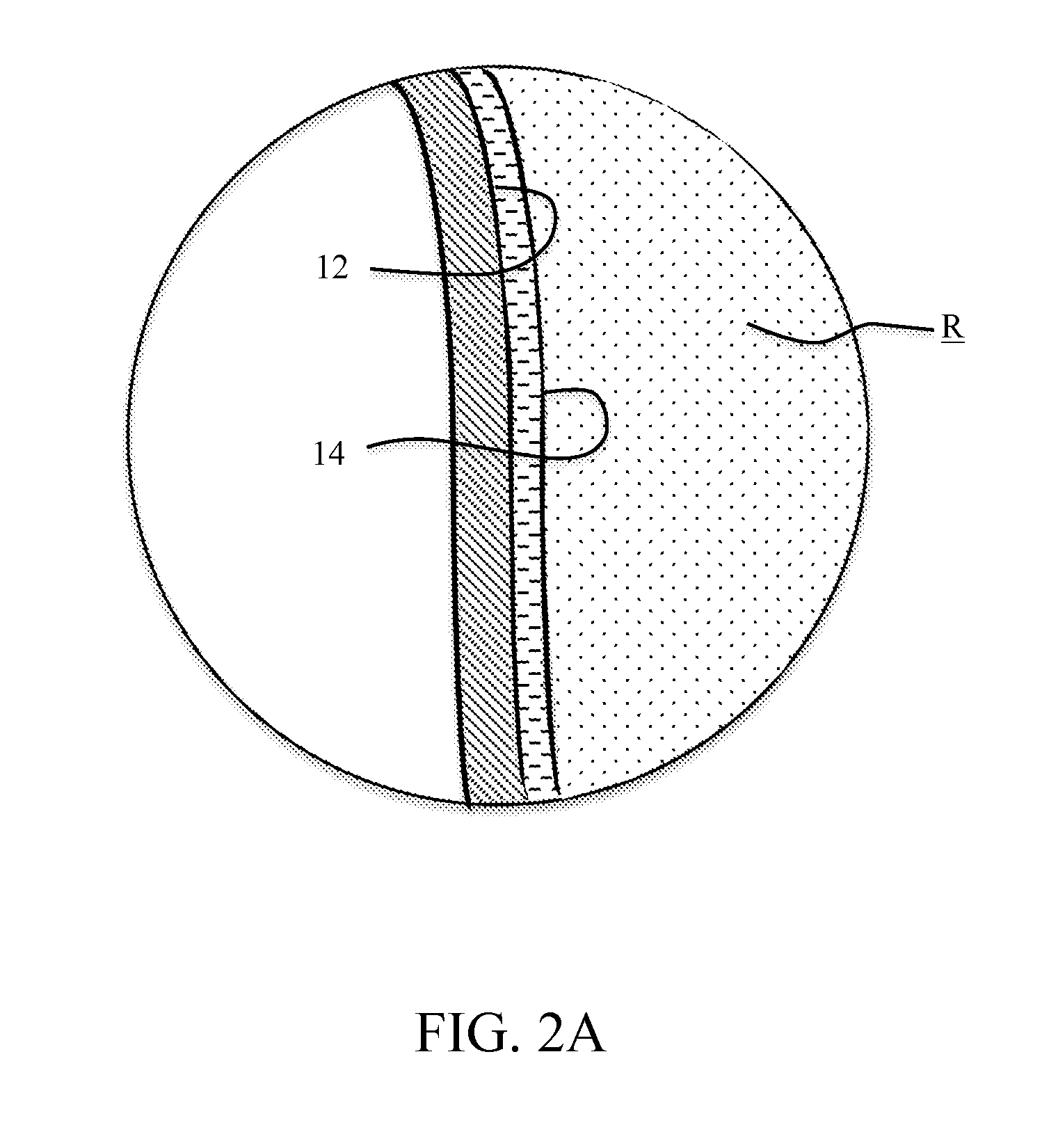
REDUCTION OF SiCl4 IN THE PRESENCE OF BCl3
ActiveUS20150265996A1Prevent/minimize silicon tetrachloride (SiCl4) formationGas treatmentSilicon halogen compoundsBoron trichlorideSilicon tetrachloride
The present invention relates, in general, to the purification of boron trichloride (BCl3). More particularly, the invention relates to a process for minimizing silicon tetrachloride (SiCl4) formation in BCl3 production and / or the removal of SiCl4 in BCl3 product stream by preventing / minimizing the silicon source in the reaction chambers. In addition, a hydride material may be used to convert any SiCl4 present to SiH4 which is easier to remove. Lastly freeze separation would replace fractional distillation to remove SiCl4 from BCl3 that has been partially purified to remove light boilers.
Owner:MATHESON TRI GAS
Method for preparing boron fertilizer
InactiveUS20120088666A1Improve the level ofFast dissolutionBiocideAmmonium nitratesDissolutionCooling down
A method for preparing a boron fertilizer, including: (1) heating boric acid to a temperature of 180-200° C., maintaining the temperature for 20-30 min for dehydration of the boric acid to yield pyroboric acid; and (2) cooling down the pyroboric acid to a temperature of 40-60° C., crushing, and screening to yield a powdered, weakly acidic, high-content boron fertilizer. The method is energy-saving, environmentally friendly, and low in cost. The resulting boron fertilizer is weakly acidic, fast in dissolution rate, and has excellent in compounding performance
Owner:UKHAN FISHERI EHGRIKALCHERAL SAJENS EHND TECH KO
Yttrium oxide composition, a method of forming the yttrium oxide composition and a method of forming a yttrium oxide layer using the same
InactiveCN101062841AHigh transparencyLow viscosityMaterial nanotechnologyTube/lamp screens manufacturePhysical chemistrySalt solution
There are provided an yttrium oxide composition having particle size of 25 nm or less, a method of preparing the same and a method of forming an yttrium oxide layer using the same. The yttrium oxide composition is prepared by dissolving an yttrium salt into a solvent to prepare an yttrium salt solution, and adding a basic compound to the yttrium salt solution to adjust a pH value to a range of 3.7 to 7. The yttrium oxide composition can form an yttrium oxide layer having improved properties since the composition has particle size of 25 nm or less and uniform particle distribution.
Owner:SAMSUNG CORNING CO LTD
Yttrium oxide composition, method of preparing the same, and method of forming yttrium oxide layer using the same
InactiveUS20070248812A1High transparencyLow viscosityMaterial nanotechnologyTube/lamp screens manufactureSolventSalt solution
There are provided an yttrium oxide composition having particle size of 25 nm or less, a method of preparing the same and a method of forming an yttrium oxide layer using the same. The yttrium oxide composition is prepared by dissolving an yttrium salt into a solvent to prepare an yttrium salt solution, and adding a basic compound to the yttrium salt solution to adjust a pH value to a range of 3.7 to 7. The yttrium oxide composition can form an yttrium oxide layer having improved properties since the composition has particle size of 25 nm or less and uniform particle distribution.
Owner:SAMSUNG CORNING CO LTD
Preparation method and application of boron oxide quantum dots
ActiveCN112250081ARapid metabolic excretionNothing producedMaterial nanotechnologyEnergy modified materialsFreeze-dryingNeutron capture
The invention relates to a preparation method of boron oxide quantum dots, and belongs to the technical field of quantum dot preparation. The method comprises the following steps: dissolving ammoniumpentaborate and boric acid in deionized water, uniformly stirring the mixture to obtain an ammonium pentaborate / boric acid mixed solution, carrying out hydrothermal reaction to obtain an initial product solution, adding a reducing agent solution, fully stirring the solution, and carrying out freeze drying to finally obtain the boron oxide quantum dot powder. The preparation method is simple, conditions are mild, no harmful by-product is generated in the preparation process, and is environmentally friendly; by optimizing the precursor, the structure of the material is controllable, and therefore, the obtained boron oxide quantum dot is uniform in size, has blue fluorescence, can be used as a boron-containing drug for boron neutron capture therapy, and has large-scale production potential and wide commercial application prospect.
Owner:ZHONGBEI UNIV
Al2O3-B2O3-SiO2 composite sol, core-shell structure activated carbon fiber and preparation method thereof
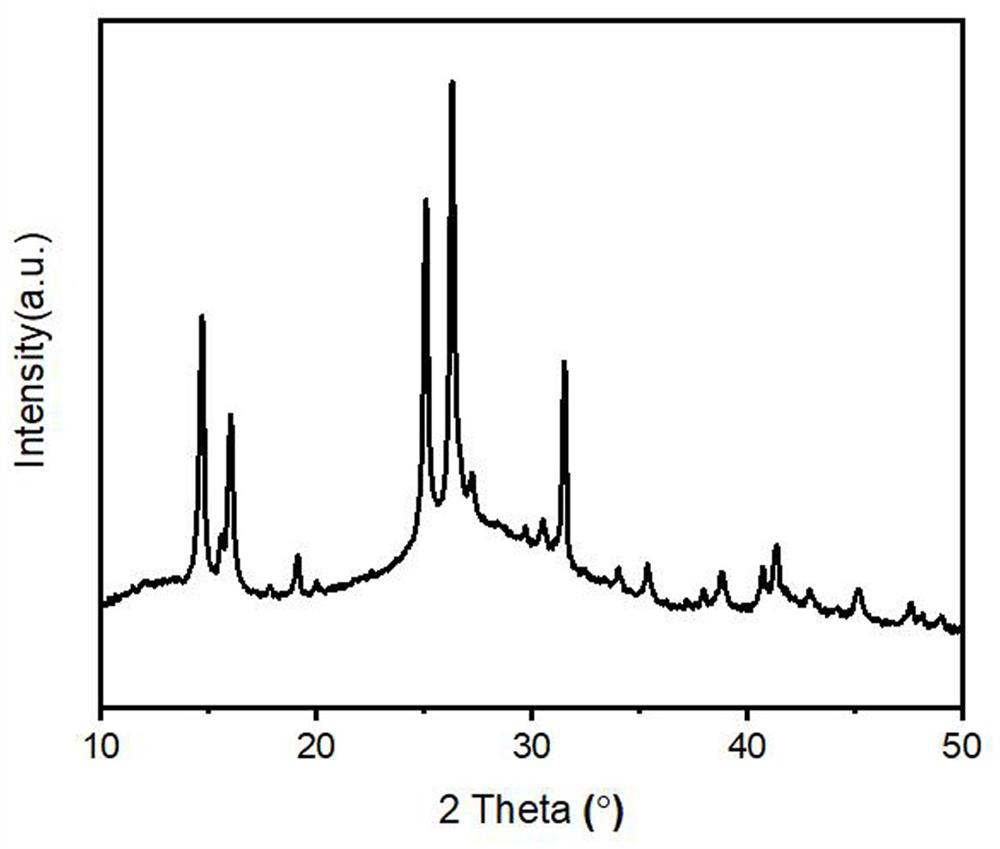
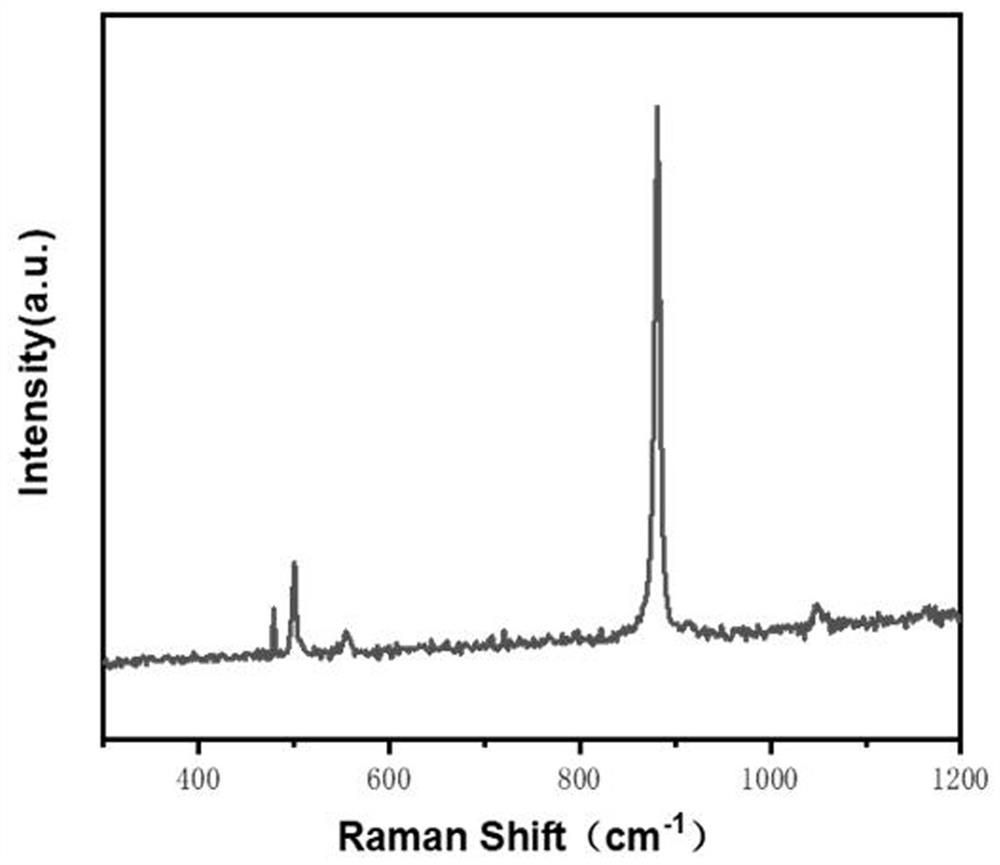
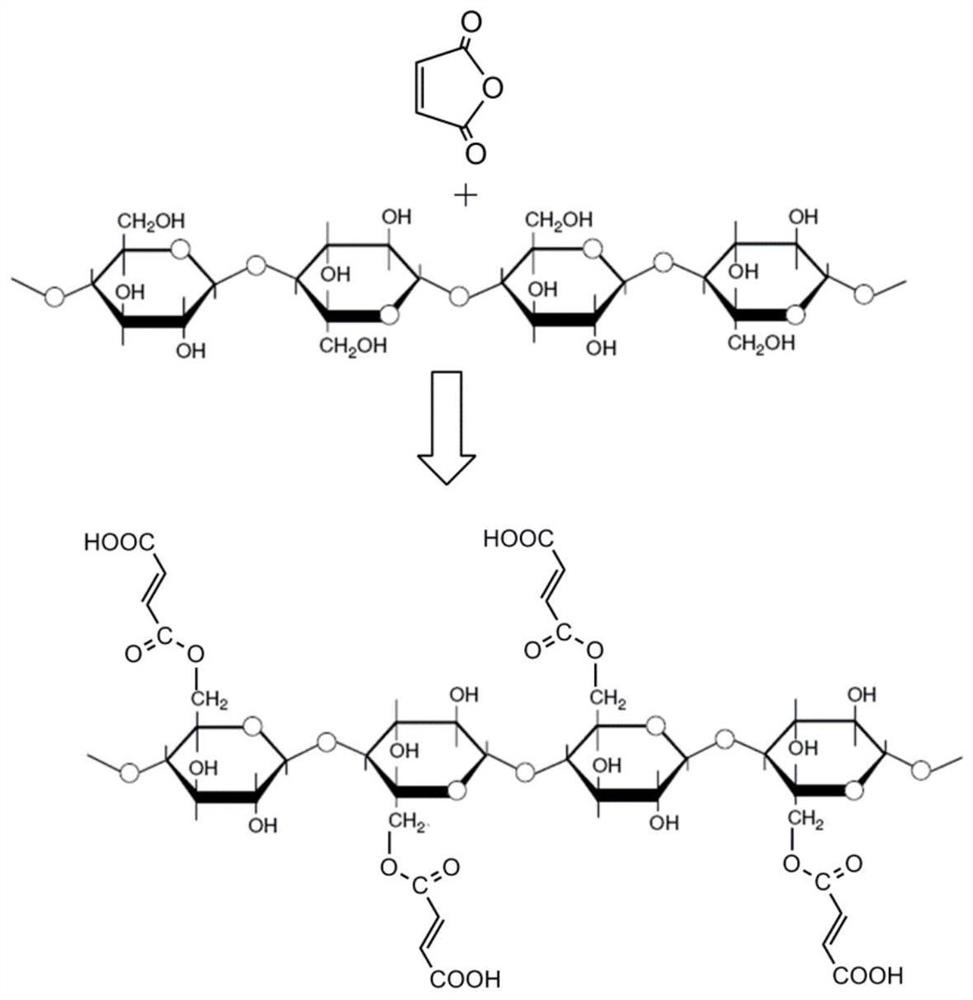
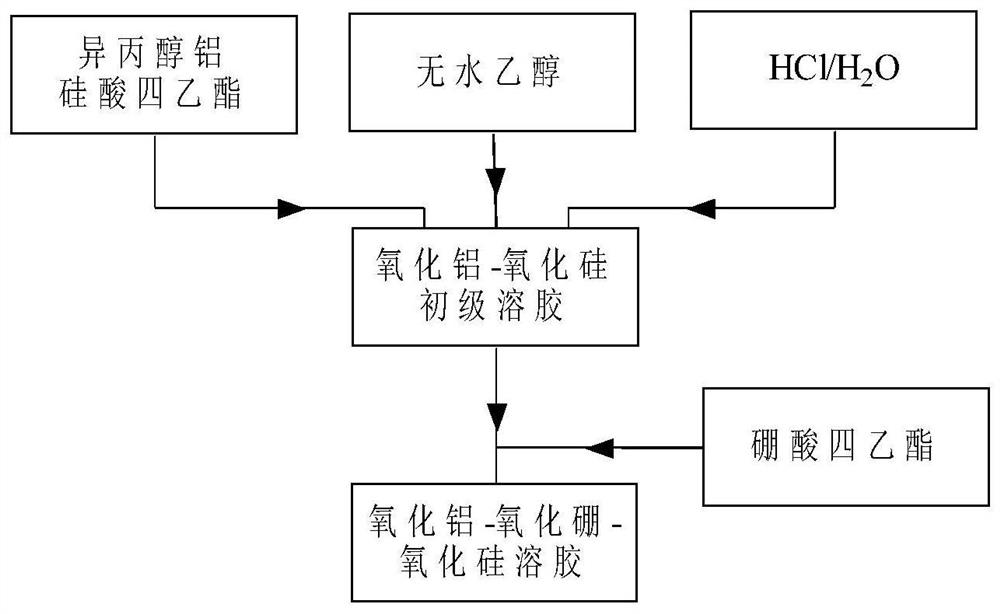
ActiveCN111617706AHigh strengthGood flexibilityOther chemical processesAluminium oxide/hydroxide preparationPolymer sciencePolymer chemistry
The invention relates to Al2O3-B2O3-SiO2 composite sol, a core-shell structure activated carbon fiber and a preparation method thereof, belongs to the technical field of activated carbon fibers, and solves the technical problem of low intrinsic strength of activated carbon fibers in the prior art. The preparation method of the core-shell structure activated carbon fiber comprises the following steps: 1, carrying out surface modification on a regenerated cellulose fiber by utilizing maleic anhydride to obtain modified regenerated cellulose fiber; 2, carrying out surface modification treatment on the modified regenerated cellulose fiber by using Al2O3-B2O3-SiO2 composite sol, and further carrying out gel curing on the obtained modification layer; and 3, carbonizing and activating the modified regenerated cellulose fiber to obtain the core-shell structure activated carbon fiber with a Al2O3-B2O3-SiO2 composite oxide modification layer. The core-shell structure activated carbon fiber provided by the invention can greatly improve the strength, flexibility and weavability of a product.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Doped and mixed nanophase material and preparation method and application thereof
InactiveCN105692630ASmall particle sizeConcentrated particle size distributionMaterial nanotechnologySilicaAll solid stateSolvent
The invention relates to a doped and mixed nanophase material and a preparation method and application thereof.The preparation method includes the steps that a powder material, a material and a dispersing agent are added to solvent and dispersed evenly, preliminary grinding and fine grinding are conducted, magnetism removing is conducted, and the doped and mixed nanophase material is obtained; the particle size of the doped and mixed nanophase material prepared through the method is 1 nm-500 nm, and the doped and mixed nanophase material can serve as the key material of a precursor of the key material of a lithium ion battery, a lithium ion capacitor, a lithium sulphur battery, a all-solid-state battery, a solar cell and the like.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI +1
Method for circularly extracting metal oxide
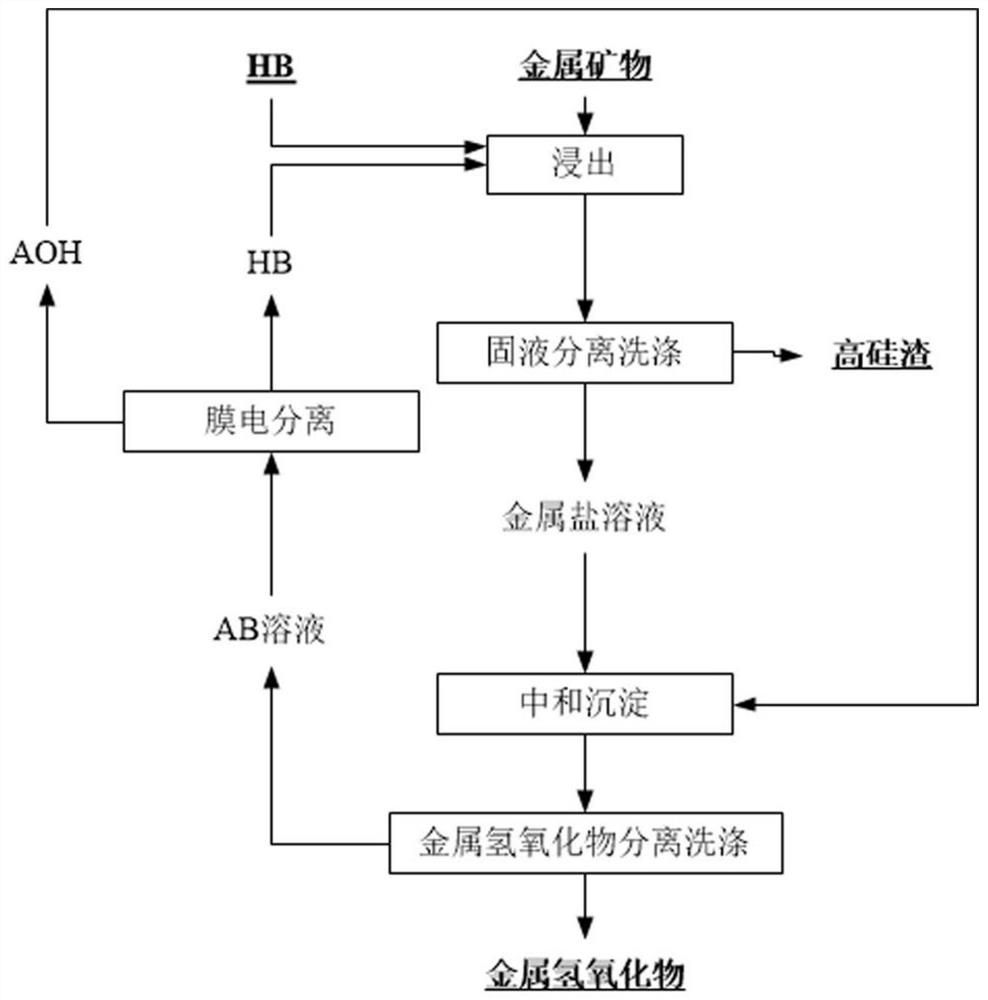
InactiveCN111961847AAchieve cycleHigh extraction rateProcess efficiency improvementBoron oxidesElectrolysisSlag
The invention relates to a method for circularly extracting metal oxide. The method comprises the steps of mixing minerals containing metal oxides with acid to obtain a mixture, and adding the mixtureinto an acid-resistant reaction kettle for reaction; carrying out solid-liquid separation and washing to obtain a metal salt solution and high-silicon slag; adding the obtained metal salt solution into an alkali solution for neutralization to obtain a metal hydroxide precipitate and a salt solution, and then carrying out solid-liquid separation and washing to wash the metal hydroxide until the metal hydroxide is neutral; collecting the obtained metal hydroxide as a product for extracting the metal oxide; separating the obtained salt solution through an electric membrane to obtain an acid solution and an alkali solution; returning the alkaline solution from the membrane electrolysis cathode area to the front steps for recycling; and concentrating the acid solution coming out of the membrane electrolysis anode area directly or through evaporation, and returning to start the recycling. According to the method, no auxiliary agent is added, so that the metal oxide in low-grade material isefficiently and effectively leached, and the extraction rate is high; and the circulation of the salt material is realized, and the discharge of waste gas, waste liquid and waste residue is avoided.
Owner:SHENYANG POLYTECHNIC UNIV
Positive active material for lithium secondary battery, electrode for lithium secondary battery, and lithium secondary battery
InactiveUS8822080B2Non-metal conductorsConductive materialHigh temperature storageLithium-ion battery
The present invention provides a Li3V2(PO4)3-based positive active material for a lithium secondary battery, which has high discharge capacity and excellent storage performance, particularly high-temperature storage performance; and a lithium secondary battery made using the positive active material. The positive active material for a lithium secondary battery has general formula Li3V2(PO4)3−x(BO3)x (0<x≦2−2). It is preferable that x be 2−7≦x≦2−3. Also provided are a positive electrode for a lithium secondary battery containing the positive active material; and a lithium secondary battery including the positive electrode.
Owner:GS YUASA INT LTD
Positive electrode active material for lithium secondary battery, electrode for lithium secondary battery, and lithium secondary battery
InactiveCN102612773AConducive to preservationCell electrodesLi-accumulatorsLithium-ion batteryChemistry
Disclosed are: a Li3V2(PO4)3-type positive electrode active material for a lithium secondary battery, which has high discharge capacity and excellent storage performance, particularly excellent storage performance at higher temperatures; and a lithium secondary battery utilizing the positive electrode active material. Specifically disclosed are: a positive electrode active material for a lithium secondary battery, which is characterized by being represented by general formula Li3V2(PO4)3-x(BO3)x (wherein x is more than 0 and not more than 2-2, preferably falls within the range from 2-7 to 2-3 inclusive); a positive electrode for a lithium secondary battery, which comprises the positive electrode active material; and a lithium secondary battery equipped with the positive electrode.
Owner:GS YUASA INT LTD
Production method of high temperature covering agent grade boron oxide
The invention relates to a production method of high temperature covering agent grade boron oxide. The production method comprises following steps: S1, boric acid heating is carried out; S2, anhydrousboron oxide is added; S3, calcining and dehydration are carried out; and S4, casting moulding is carried out. According to the production method, comprehensive consideration of production cost, production period, and product quality stability is adopted, the lowest vacuum degree and the highest dehydration temperature are not adopted, the boron oxide capable of satisfying high temperature covering grade requirements is obtained, and product yield is 95% or higher.
Owner:广东先导微电子科技有限公司
Mesoporous metal oxides and processes for preparation thereof
A process for preparing a mesoporous metal oxide, i.e., transition metal oxide. Lanthanide metal oxide, a post-transition metal oxide and metalloid oxide. The process comprises providing an acidic mixture comprising a metal precursor, an interface modifier, a hydrotropic ion precursor, and a surfactant; and heating the acidic mixture at a temperature and for a period of time sufficient to form the mesoporous metal oxide. A mesoporous metal oxide prepared by the above process. A method of controlling nano-sized wall crystallinity and mesoporosity in mesoporous metal oxides. The method comprises providing an acidic mixture comprising a metal precursor, an interface modifier, a hydrotropic ion precursor, and a surfactant; and heating the acidic mixture at a temperature and for a period of time sufficient to control nano-sized wall crystallinity and mesoporosity in the mesoporous metal oxides. Mesoporous metal oxides and a method of tuning structural properties of mesoporous metal oxides.
Owner:UNIV OF CONNECTICUT
Boron suboxide composite material
InactiveUS20120217436A1Readily brazeableReadily brazeable to substrateOther chemical processesAbrasion apparatusAlloyCopper
The invention provides a boron suboxide composite material comprising boron suboxide and a secondary phase, wherein the secondary phase contains a metal selected from the group of gold, silver and copper and alloys based on or containing one or more of these metals. Moreover, the metal or alloy is present in the material in an amount of less than about 20 volume %, and preferably less than about 6 volume %.
Owner:ELEMENT SIX ABRASIVES
Boron suboxide composite material
Owner:ELEMENT SIX ABRASIVES
Carbon combustion synthesis of oxides
ActiveUS7897135B2Low production costWell mixedAlkaline earth titanatesPigmenting treatmentSelf-propagating high-temperature synthesisCeramic composite
The present invention is generally directed to a novel, economic synthesis of oxide ceramic composites. Methods of the present invention, referred to as carbon combustion synthesis of oxides (CCSO), are a modification of self-propagating high-temperature synthesis (SHS) methods in which the heat needed for the synthesis is generated by combustion of carbon in oxygen rather than that of a pure metal. This enables a more economic production of the ceramic material and minimizes the presence of intermediate metal oxides in the product. The reactant mixture generally comprises at least one oxide precursor (e.g., a metal or non metal oxide, or super oxide, or nitride, or carbonate, or chloride, or oxalate, or halides) as a reactant, but no pure metal. Pure carbon in the form of graphite or soot is added to the reactant mixture to generate the desired heat (upon ignition). The mixture is placed in a reactor and exposed to gaseous oxygen. The high-temperature exothermic reaction between the carbon and oxygen generates a self-sustaining reaction in the form of a propagating temperature wave that causes a reaction among the reactants. The reaction proceeds rapidly following ignition, and the final product comprises simple and / or complex oxides of elements present in the oxide precursor(s). CCSO also enables synthesis of oxides that cannot be produced by conventional SHS, such as when the pure metal is pyrophoric (such as Li or La) or such as when it melts at room temperature (e.g., Ga) or such as the combustion heat of the metal is relatively low.
Owner:UNIV HOUSTON SYST
Quaternary positive electrode material and preparation method and application thereof
ActiveCN113582253AImprove cycle lifeMaintain high nickel capacitySecondary cellsPositive electrodesLithiumNiobium
The invention provides a quaternary positive electrode material and a preparation method and an application thereof, and the preparation method comprises the following steps: (1) mixing a quaternary precursor, a niobium source, a boron source and a lithium source, and carrying out primary calcination to obtain a boron / niobium doped quaternary positive electrode material; and (2) washing the boron / niobium doped quaternary positive electrode material obtained in the step (1) with water, drying, mixing with boric acid, and carrying out secondary calcination to obtain the quaternary positive electrode material, wherein the chemical formula of the quaternary precursor in the step (1) is LiNixCoyMnzAl(1-x-y-z)O2(x is greater than or equal to 0.9 and less than 1, y is greater than 0 and less than 0.07, and z is greater than 0 and less than 0.03). According to the preparation method disclosed by the invention, the lattice structure is stabilized and the generation of microcracks is limited by adjusting the textured microstructure of the ultrahigh nickel positive electrode material through a niobium / boron co-doping mechanism, so that the cycle life of the ultrahigh nickel positive electrode material is prolonged.
Owner:SVOLT ENERGY TECHNOLOGY CO LTD
Method for preparing powdery 5N high-purity diboron trioxide
The present invention relates to a preparation method of powdery 5N high-purity boron trioxide, the steps of which are: (1) hydrolysis: in a clean 50 ml platinum crucible, add 20 grams of high-purity trimethyl borate, and then add dropwise under stirring 5 grams of pure water, continue to stir for 5 minutes after adding, and the hydrolysis reaction is completed; (2) finished product: move the platinum crucible containing the reactant into a clean quartz tube, put the quartz tube into a tube furnace, and heat up to Keep at 100°C for 2 hours, take out the platinum crucible and stir the boron trioxide in the platinum crucible with a clean polytetrafluoroethylene rod to make the boron trioxide heated more evenly in the crucible, then move the platinum crucible back into the quartz tube, Continue to raise the temperature to 200°C for 2 hours under vacuum, then cool to room temperature, take out the platinum crucible and store it in a desiccator to obtain the finished powdery high-purity boron trioxide.
Owner:天津市风船化学试剂科技有限公司
Additive Composition And Composition Binding Agent For Superhard Material And Preparation Thereof, And Self-Sharpening Diamond Grinding Wheel And Preparation Thereof
ActiveUS20180185984A1Low level of fire resistanceImprove efficiencySilicaZinc oxides/hydroxidesSuperhard materialPhysical chemistry
Disclosed are an additive raw material composition and an additive for superhard material product, a method for preparing the additive, a composite binding agent, a superhard material product, a self-sharpening diamond grinding wheel and a method for manufacturing the same. The raw material composition consisting of components in following mass percentage: Bi2O3 25%˜40%, B2O3 25%˜40%, ZnO 5%˜25%, SiO2 2%˜10%, Al2O3 2%˜10%, Na2CO3 1%˜5%, Li2CO3 1%˜5%, MgCO3 0%˜5%, and CaF2 1%˜5%. The composite binding agent is prepared from the additive and a metal composite binding agent. The self-sharpening diamond grinding wheel prepared from the composite binding agent has high self-sharpness, high strength, and fine texture, is uniformly consumed during the grinding process, does not need to be trimmed during the process of being used, and maintains good grinding force all the time, fundamentally solving the problems of long trimming time and high trimming cost of the diamond grinding wheel.
Owner:ZHENGZHOU RES INST FOR ABRASIVES & GRINDING CO LTD
Modified lithium ferric manganese phosphate material, and preparation method and application thereof
ActiveCN113224278AImprove conductivityImprove diffusion abilitySecondary cellsPositive electrodesDischarge efficiencyMagnesium doping
The invention provides a modified lithium ferric manganese phosphate material, and a preparation method and application thereof. The modified lithium iron manganese phosphate material comprises a magnesium-doped lithium iron manganese phosphate nuclear layer and a boron-containing coating layer coating the surface of the magnesium-doped lithium iron manganese phosphate nuclear layer. The modified lithium iron manganese phosphate material comprises a magnesium-doped lithium iron manganese phosphate nuclear layer and a boron-containing coating layer coating the surface of the magnesium-doped lithium iron manganese phosphate nuclear layer. Based on the structure, the material is higher in conductivity and lithium ion diffusivity, lower in charge transfer impedance and better in stability. When the composite material is used as a positive electrode material of a lithium ion battery, the rate capability of the battery is better, the first charge-discharge efficiency and capacity are higher, the cycle performance is better, and especially the cycle life is longer.
Owner:SVOLT ENERGY TECHNOLOGY CO LTD
Method for preparing boron fertilizer
InactiveUS8946120B2Low production costShorten production timeBiocideAmmonium nitratesDissolutionCooling down
A method for preparing a boron fertilizer, including: (1) heating boric acid to a temperature of 180-200° C., maintaining the temperature for 20-30 min for dehydration of the boric acid to yield pyroboric acid; and (2) cooling down the pyroboric acid to a temperature of 40-60° C., crushing, and screening to yield a powdered, weakly acidic, high-content boron fertilizer. The method is energy-saving, environmentally friendly, and low in cost. The resulting boron fertilizer is weakly acidic, fast in dissolution rate, and has excellent in compounding performance
Owner:UKHAN FISHERI EHGRIKALCHERAL SAJENS EHND TECH KO
a kind of al 2 o 3 -b 2 o 3 -sio 2 Composite sol, activated carbon fiber with core-shell structure and preparation method thereof
ActiveCN111617706BHigh strengthGood flexibilityOther chemical processesAluminium oxide/hydroxide preparationPolymer sciencePolymer chemistry
The invention relates to Al2O3-B2O3-SiO2 composite sol, a core-shell structure activated carbon fiber and a preparation method thereof, belongs to the technical field of activated carbon fibers, and solves the technical problem of low intrinsic strength of activated carbon fibers in the prior art. The preparation method of the core-shell structure activated carbon fiber comprises the following steps: 1, carrying out surface modification on a regenerated cellulose fiber by utilizing maleic anhydride to obtain modified regenerated cellulose fiber; 2, carrying out surface modification treatment on the modified regenerated cellulose fiber by using Al2O3-B2O3-SiO2 composite sol, and further carrying out gel curing on the obtained modification layer; and 3, carbonizing and activating the modified regenerated cellulose fiber to obtain the core-shell structure activated carbon fiber with a Al2O3-B2O3-SiO2 composite oxide modification layer. The core-shell structure activated carbon fiber provided by the invention can greatly improve the strength, flexibility and weavability of a product.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Preparation method of regenerated ternary positive electrode material of lithium nickel cobalt oxide battery
PendingCN113880100ASolve complexitySolve benefitsPositive electrodesWaste accumulators reclaimingElectrical batteryPhysical chemistry
The invention discloses a preparation method of a regenerated ternary positive electrode material of a lithium nickel cobalt oxide battery. The method comprises the following steps: (1) discharging waste lithium ion batteries by adopting a sodium chloride solution, conducting disassembling, soaking a positive plate in alkali liquor, and conducting filtering to obtain black powder; (2) carrying out reduction roasting on the obtained black powder in a protective gas atmosphere; (3) dissolving the black powder in an acid solution, and conducting extraction and impurity removal, so as to obtain a high-purity mixed solution containing nickel and cobalt; (4) after the concentrations of cobalt and nickel ions are measured, adding a corresponding manganese source and a corresponding tungsten source into the solution, adjusting the pH value, and carrying out a coprecipitation reaction to obtain a precursor; and (5) mixing and sintering the precursor, a lithium source and a boron source to obtain the positive electrode material. According to the method provided by the invention, not only is pollution generated by the waste lithium ion battery effectively reduced, but also the waste lithium nickel cobalt oxide material can be recycled and regenerated into the ternary positive electrode material, and the positive electrode material has excellent electrochemical performance.
Owner:CENT SOUTH UNIV
Popular searches
Group 1/11 element organic compounds Group 8/9/10/18 element organic compounds Molecular-sieve and base-exchange compounds Titanium organic compounds Group 3/13 element organic compounds Group 2/12 organic compounds without C-metal linkages Metallocenes Electrolytes Composite electrolytes Aluminium compounds
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com