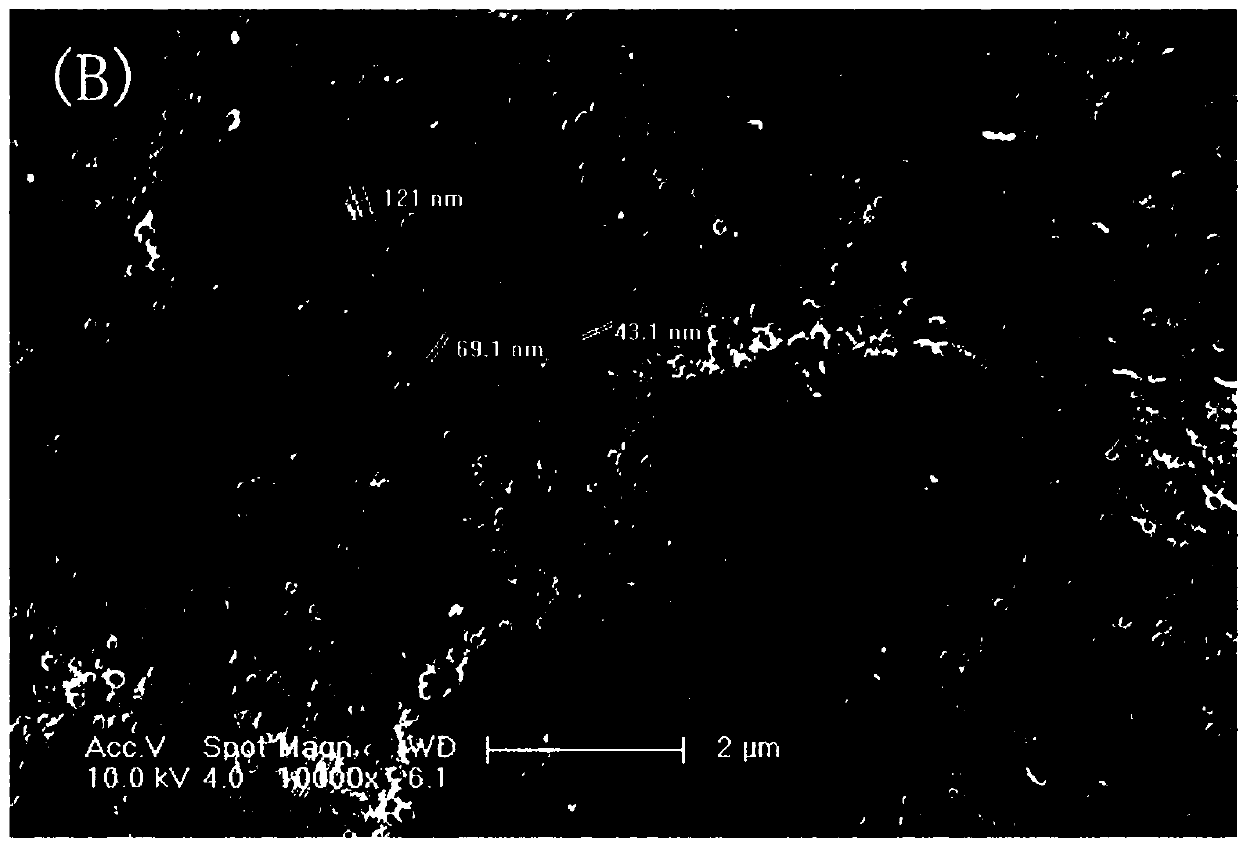
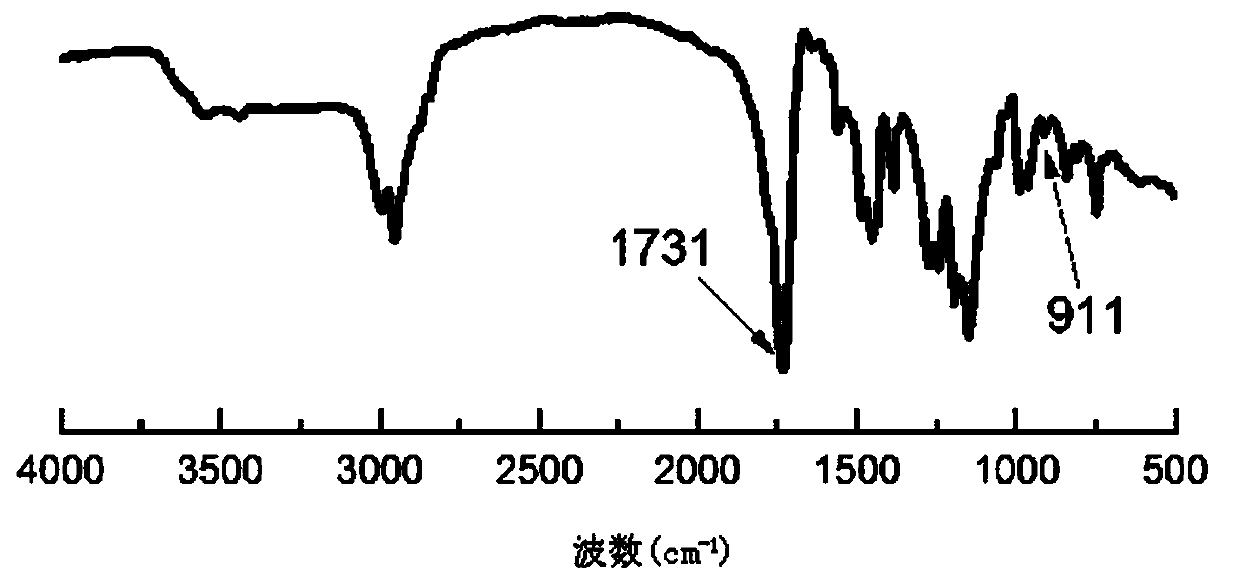
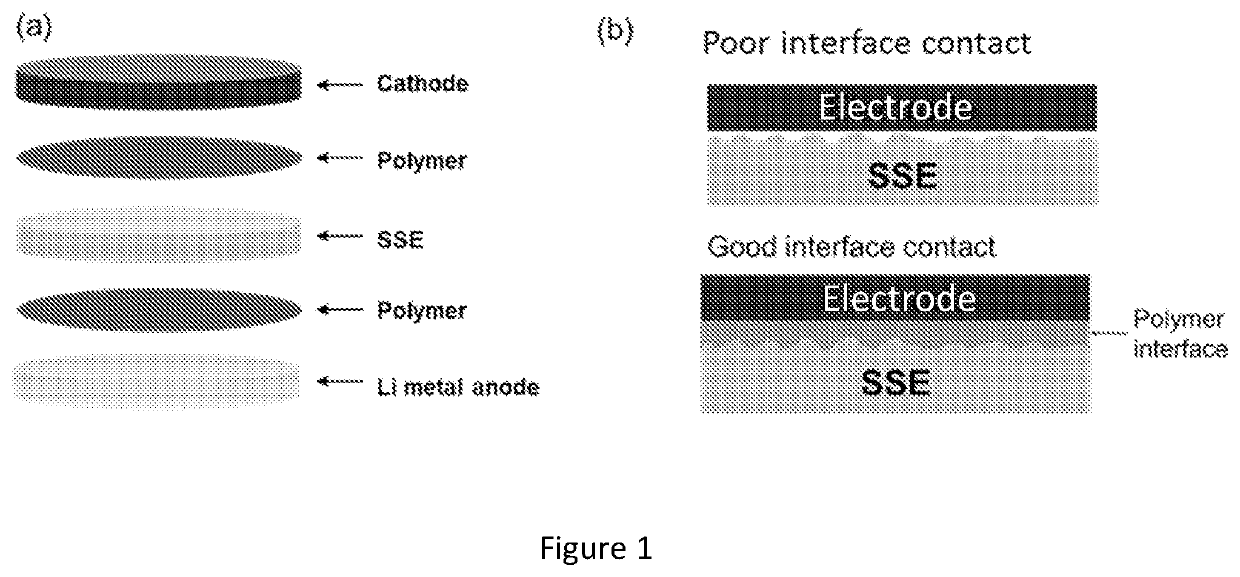
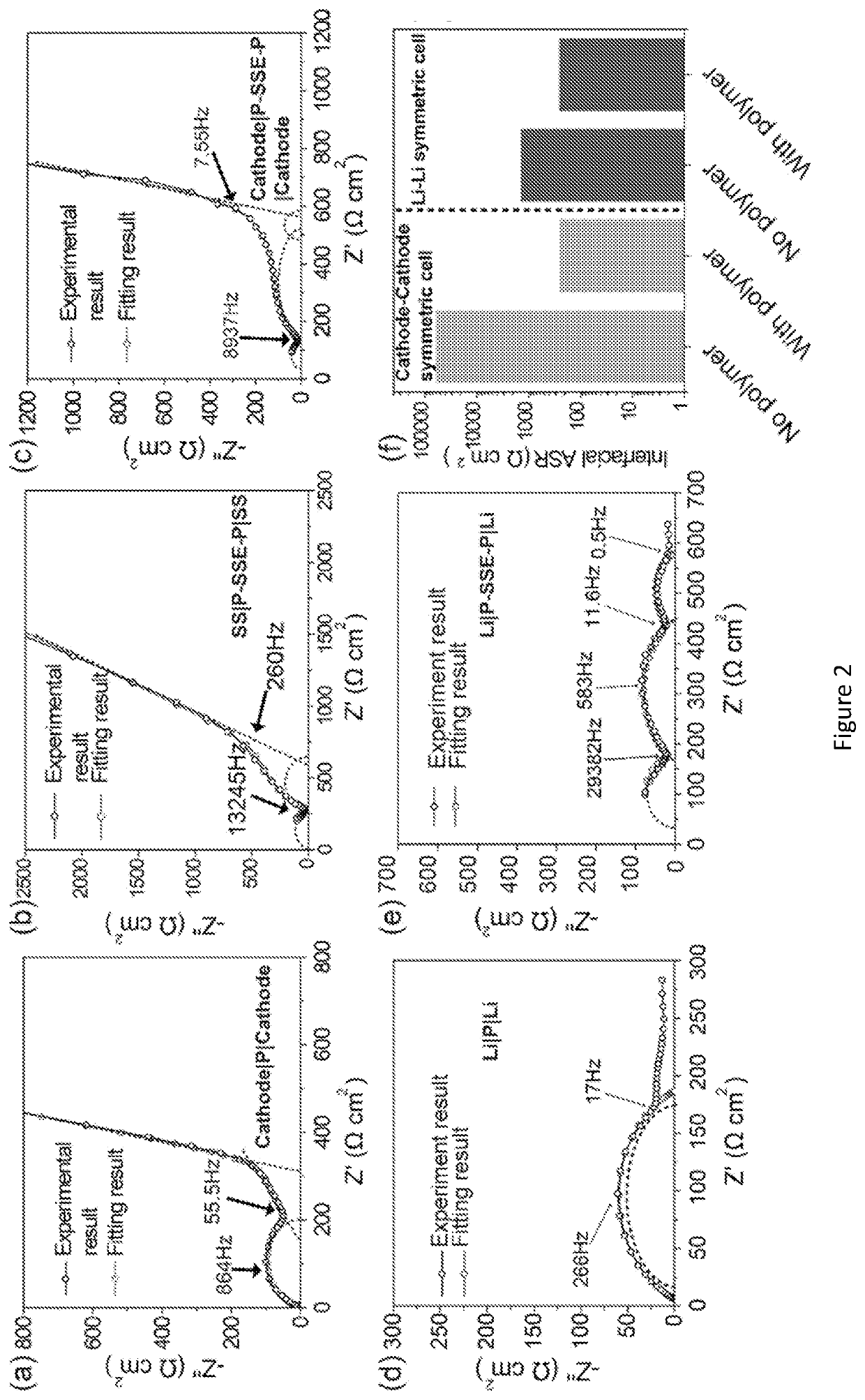
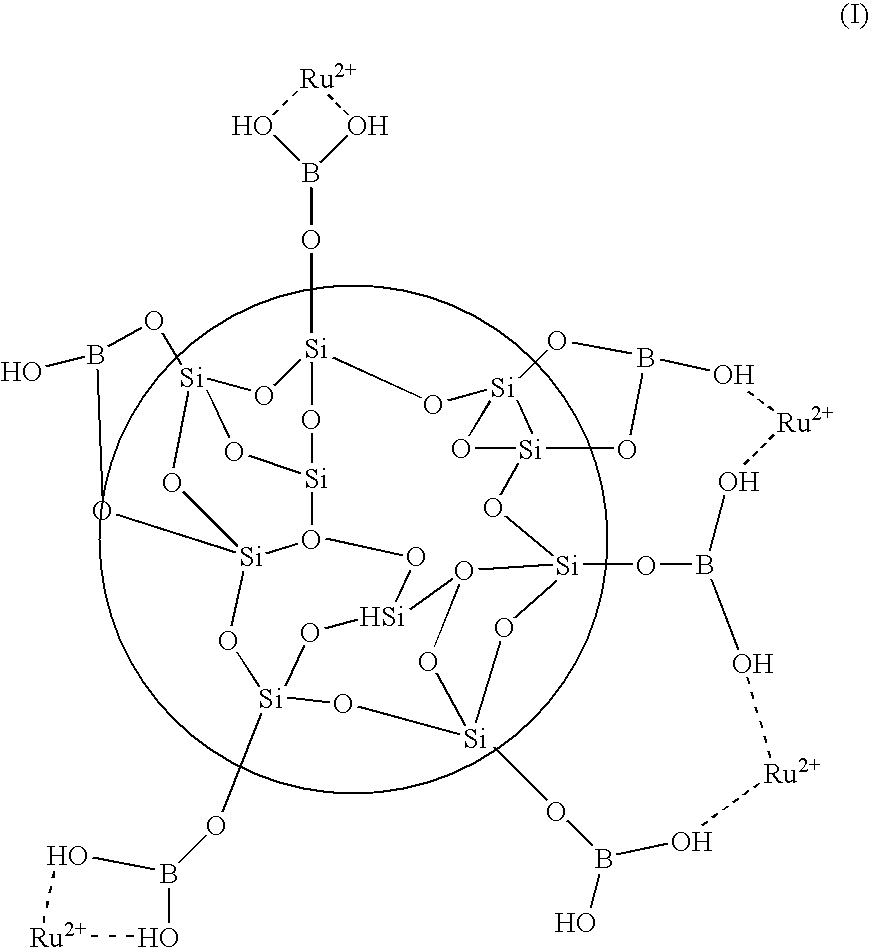
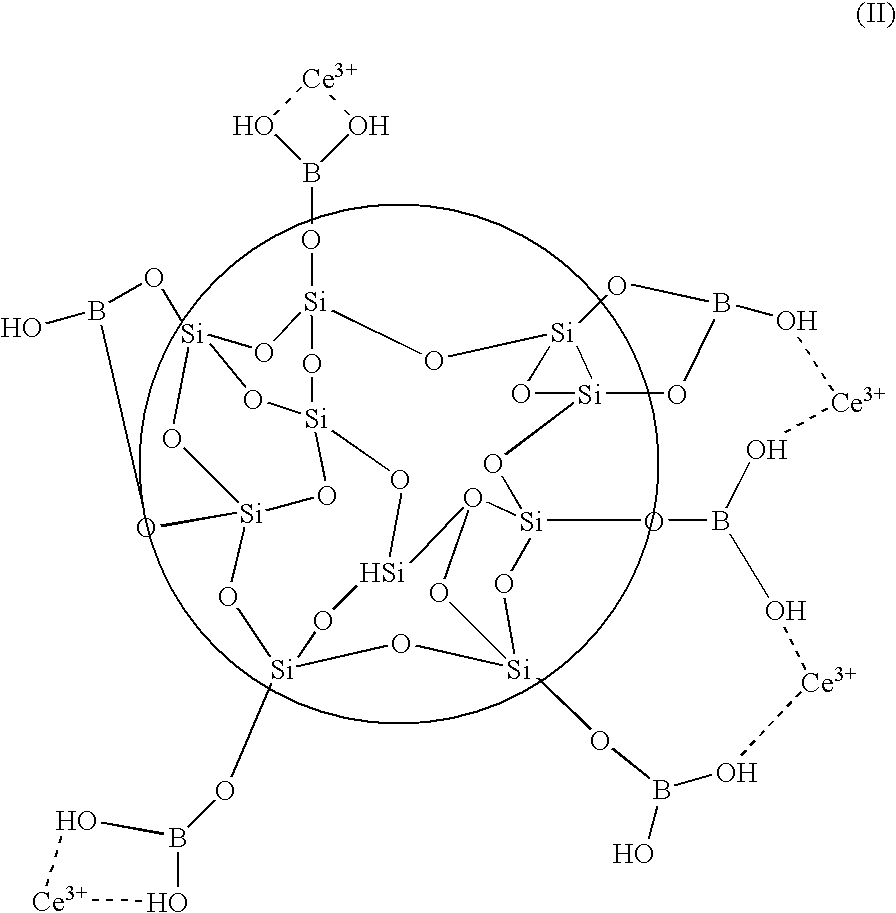
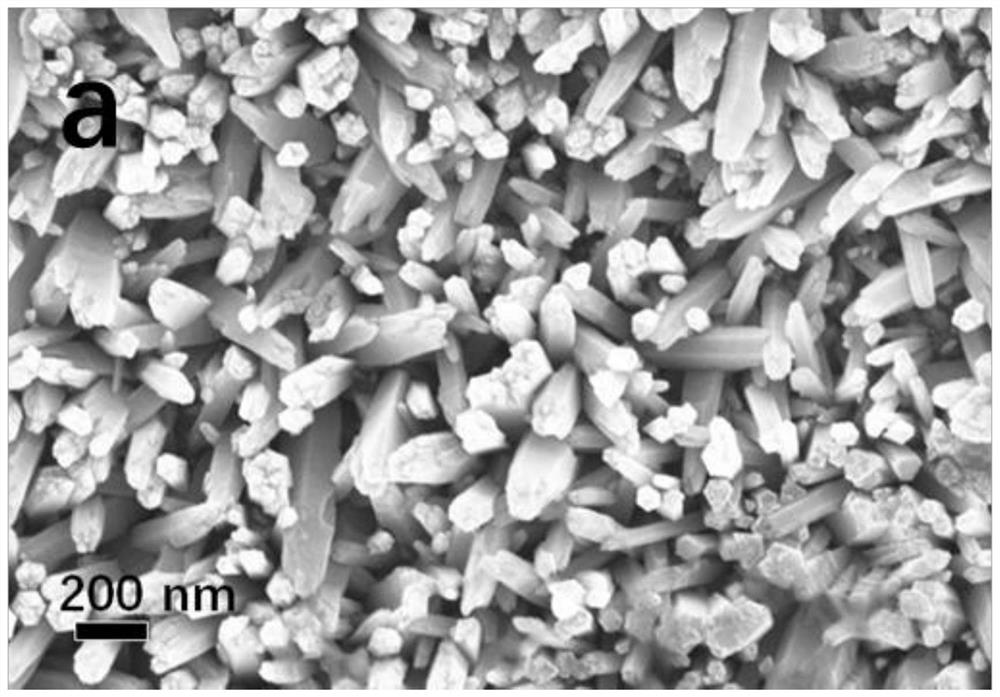
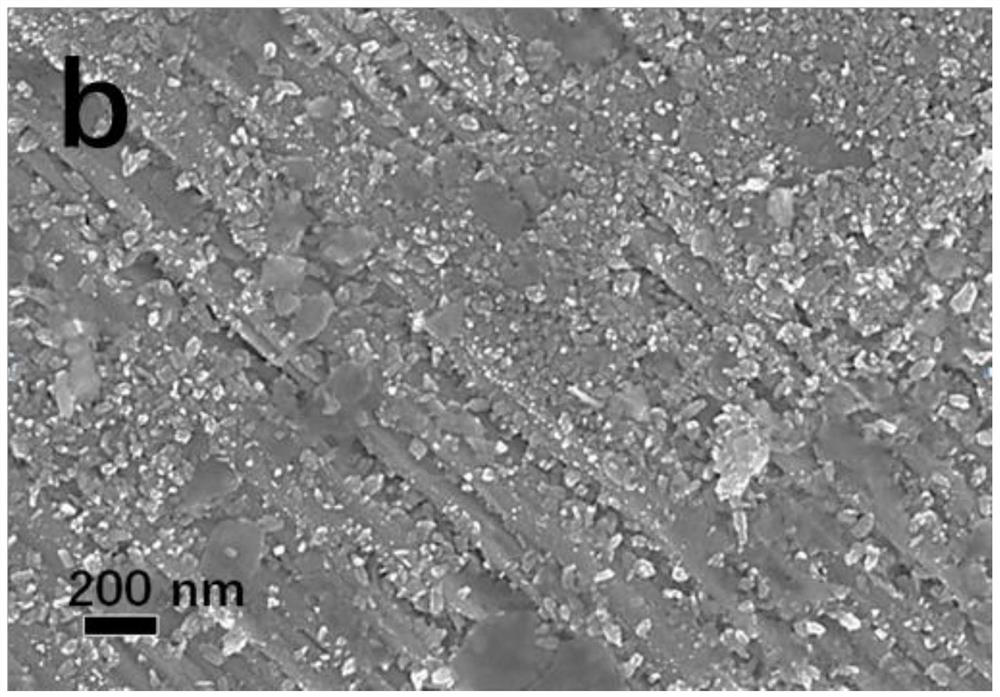
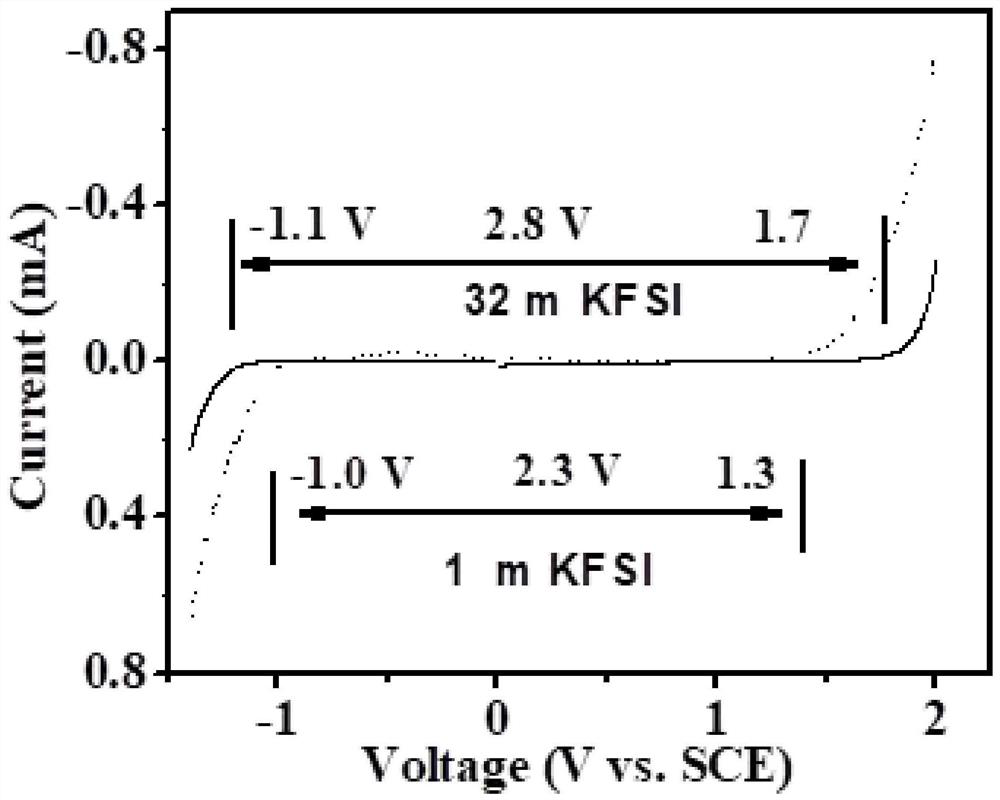
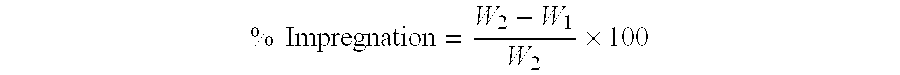Patents
Literature
529results about "Composite electrolytes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
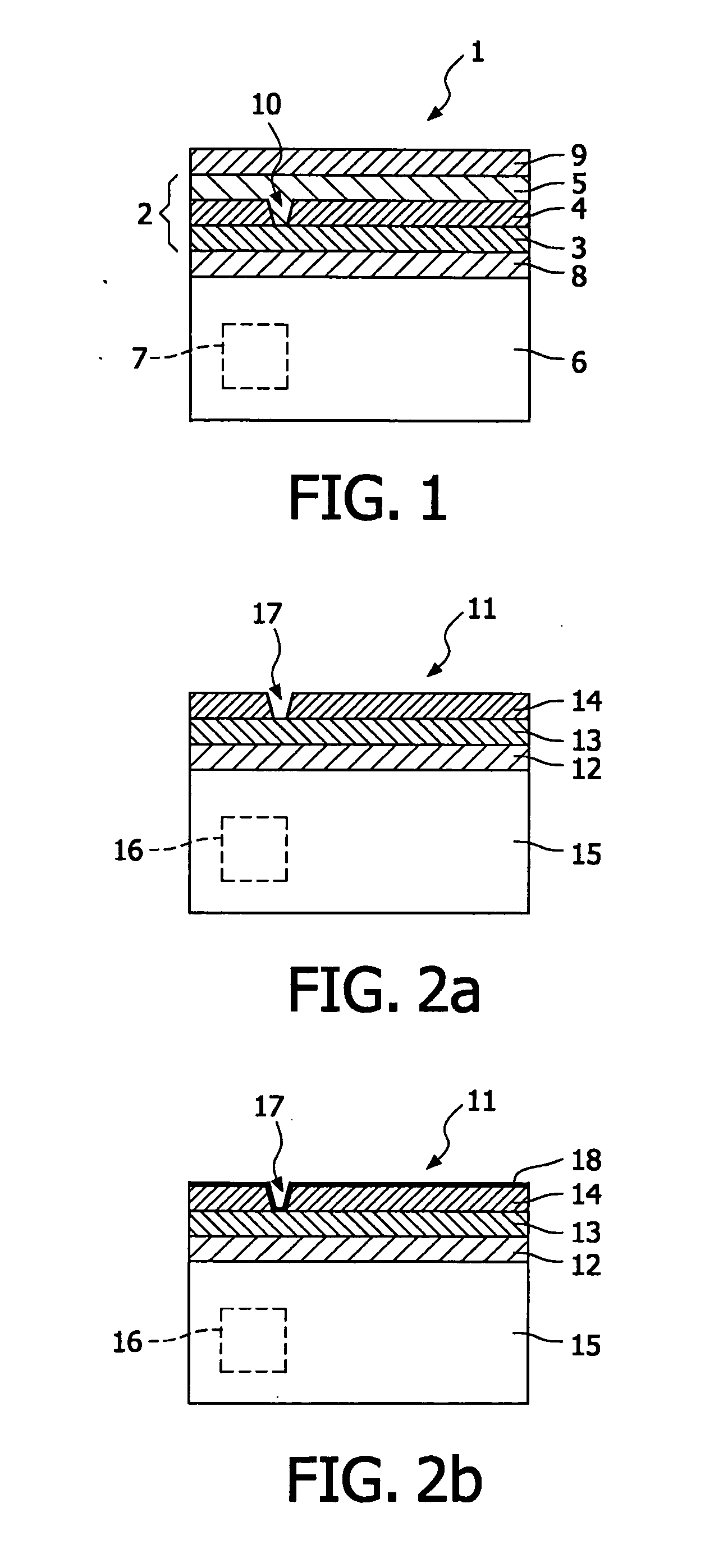
Solid state electrolyte and electrode compositions
ActiveUS20150188187A1Large capacityLower impedanceSolid electrolytesSolid electrolyte cellsSolid state electrolyteLithium-ion battery
A lithium ion battery having an anode, a solid electrolyte, and a cathode. The cathode includes an electrode active material, a first lithium salt, and a polymer material. The solid electrolyte can include a second lithium salt. The solid electrolyte can include a ceramic material, a lithium salt, and a polymer material.
Owner:WILDCAT DISCOVERY TECH
Solid electrolyte material manufacturable by polymer processing methods
ActiveUS8268197B2Improve Li-based batteriesIncrease energy densityConductive materialSolid electrolyte cellsPolymer electrolytesHigh energy
The present invention relates generally to electrolyte materials. According to an embodiment, the present invention provides for a solid polymer electrolyte material that is ionically conductive, mechanically robust, and can be formed into desirable shapes using conventional polymer processing methods. An exemplary polymer electrolyte material has an elastic modulus in excess of 1×106 Pa at 90 degrees C. and is characterized by an ionic conductivity of at least 1×10−5 Scm-1 at 90 degrees C. An exemplary material can be characterized by a two domain or three domain material system. An exemplary material can include material components made of diblock polymers or triblock polymers. Many uses are contemplated for the solid polymer electrolyte materials. For example, the present invention can be applied to improve Li-based batteries by means of enabling higher energy density, better thermal and environmental stability, lower rates of self-discharge, enhanced safety, lower manufacturing costs, and novel form factors.
Owner:RGT UNIV OF CALIFORNIA +1
Ceramic-ceramic nanocomposite electrolyte
ActiveUS20050214616A1Improve conductivityImprove ionic conductivityMaterial nanotechnologyFinal product manufactureDopantComposite electrolyte
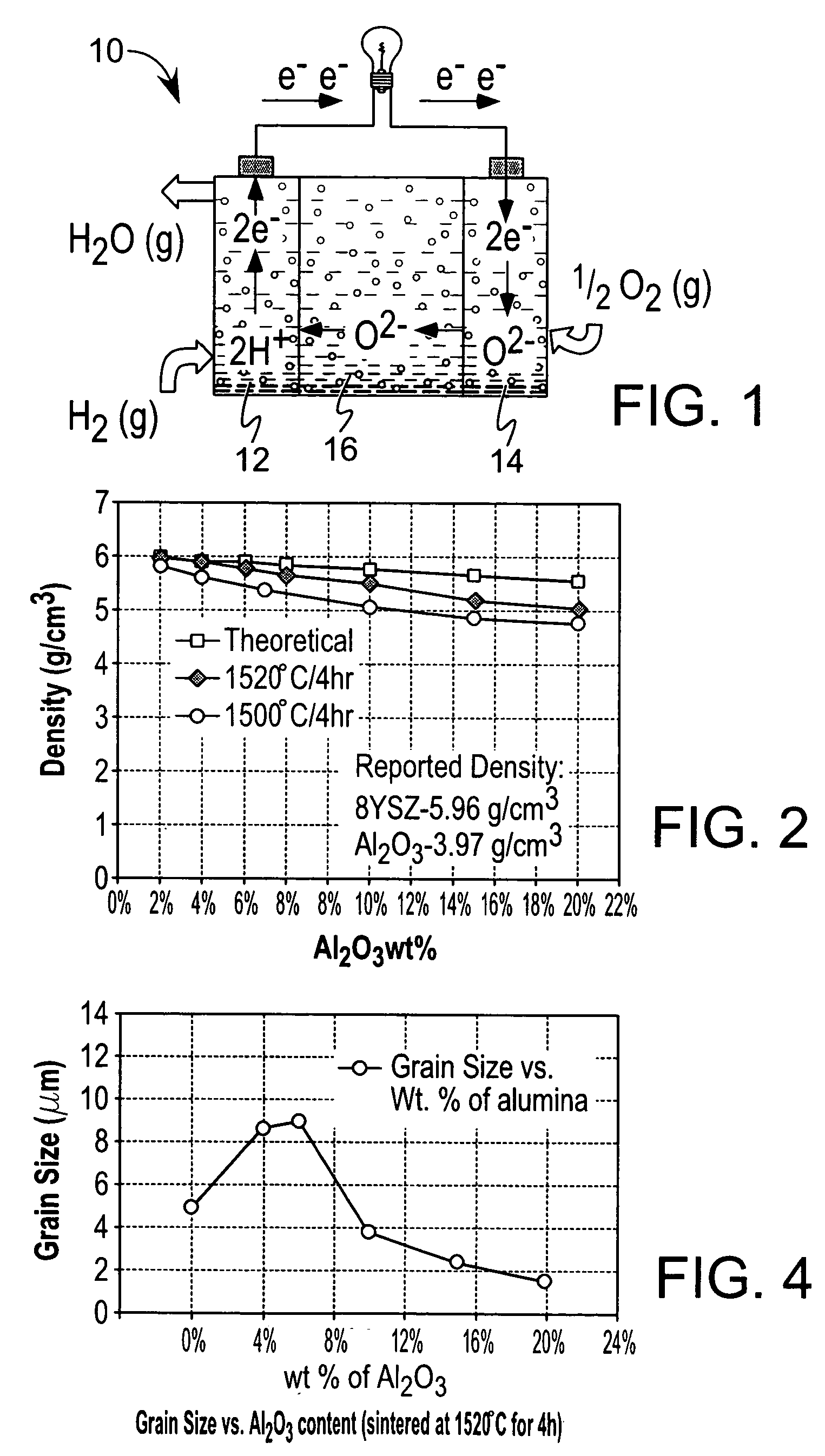
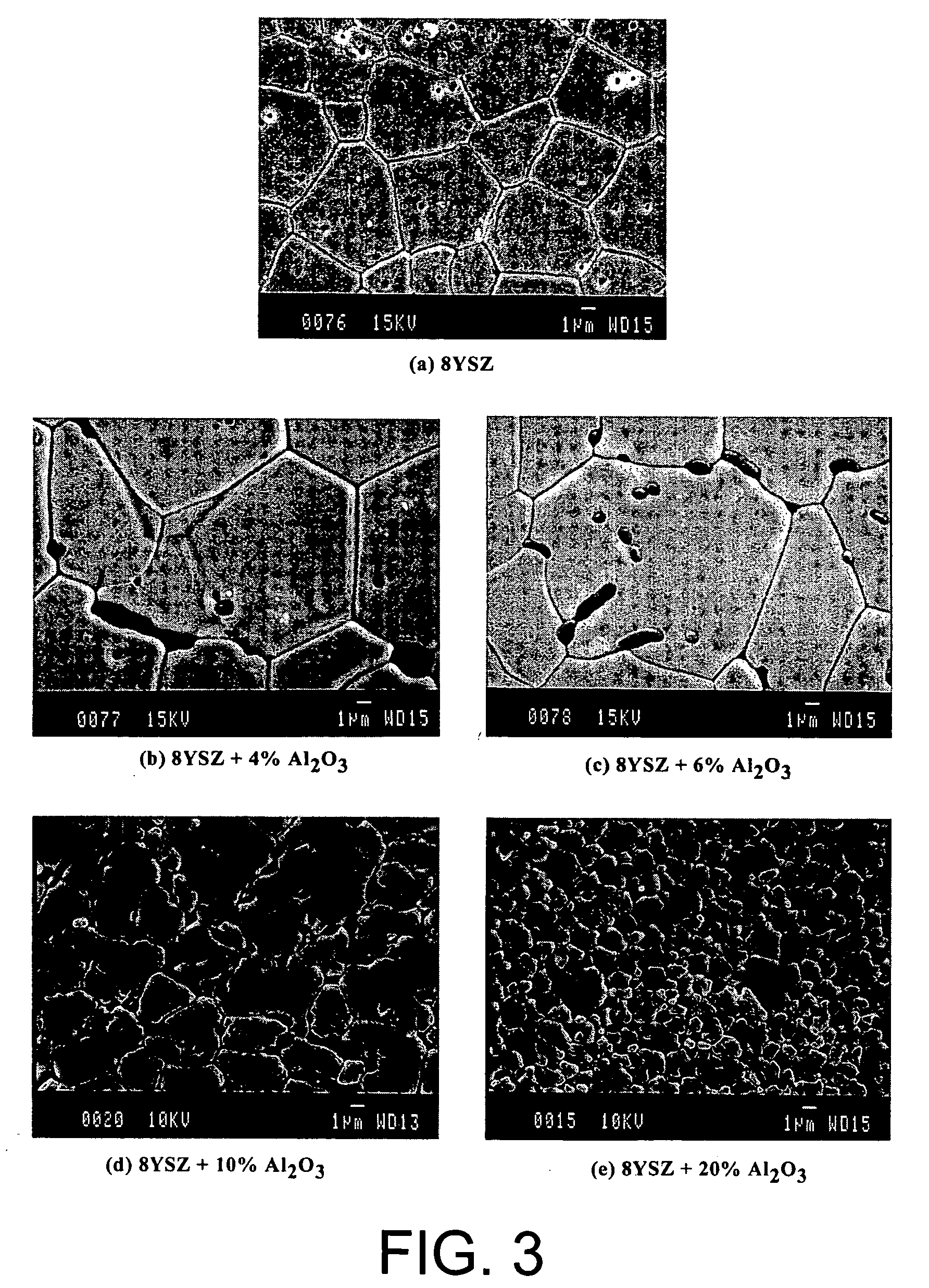
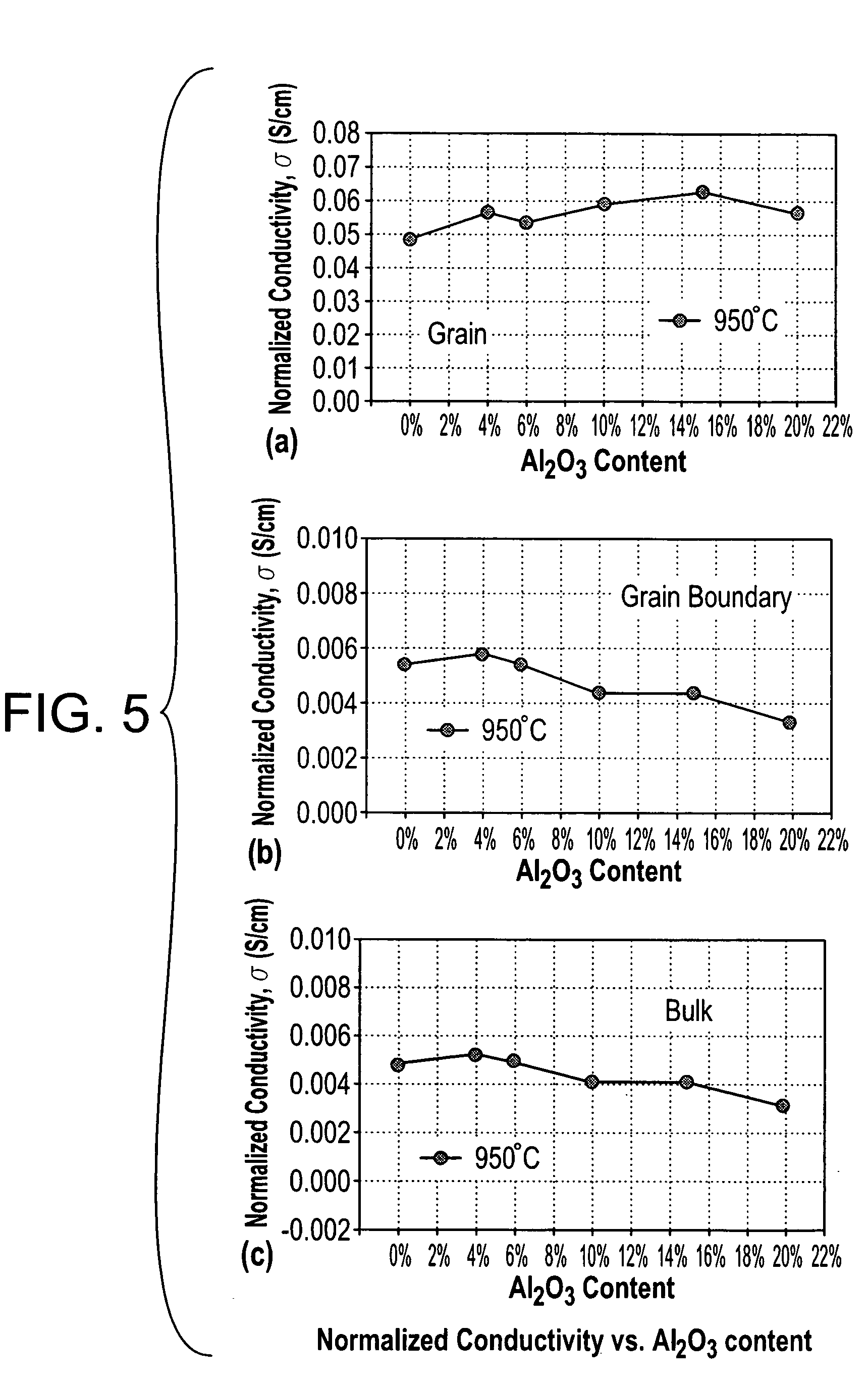
A ceramic-ceramic nanocomposite electrolyte having enhanced conductivity is provided. The nancomposite electrolyte is formed from chemically stabilized zirconia such as yttria stabilized zirconia or scandia stabilized zirconia and a heterogeneous ceramic dopant material such as Al2O3, TiO2, MgO, BN, or Si3N4 The nanocomposite electrolyte is formed by doping the chemically stabilized zirconia with the ceramic dopant material and pressing and sintering the composite. The resulting electrolyte has a bulk conductivity of from about 0.10 to about 0.50 S / cm at about 600° C. to about 900° C. and may be incorporated into a solid oxide fuel cell.
Owner:UNIV OF DAYTON
All-solid-state battery containing nano-solid electrolyte and method of manufacturing the same
Provided is an all-solid-state battery containing a nano-solid electrolyte which has excellent stability and enhanced battery performance and can be manufactured without changing an existing process, and a method of manufacturing the same. Due to substantially improved battery performance, as well as having excellent safety, the all-solid-state battery containing a nano-solid electrolyte may be widely used and further contribute to industrial development such as electric vehicles in which medium and large lithium ion rechargeable batteries are used.
Owner:HYUNDAI MOTOR CO LTD
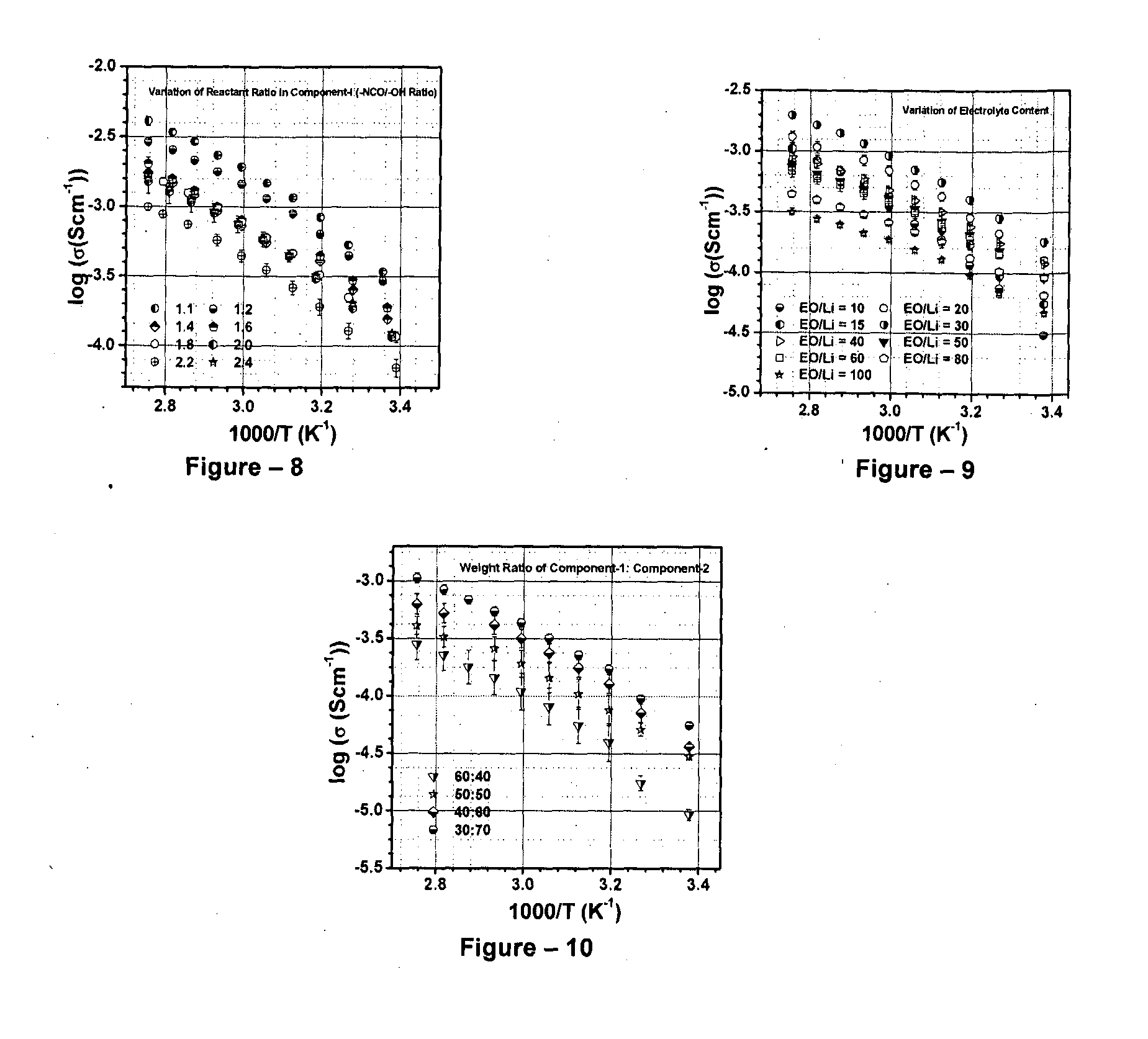
High-ionic conductivity electrolyte compositions comprising semi-interpenetrating polymer networks and their composites
InactiveUS20160049690A1Improve ionic conductivityReduce crystallinitySolid electrolytesLight-sensitive devicesEnd-groupPolymer network
The invention relates to high-ionic conductivity electrolyte compositions. The invention particularly relates to high-ionic conductivity electrolyte compositions of semi-interpenetrating polymer networks and their nanocomposites as quasi-solid / solid electrolyte matrix for energy generation, storage and delivery devices, in particular for hybrid solar cells, rechargeable batteries, capacitors, electrochemical systems and flexible devices. The binary or ternary component semi-interpenetrating polymer network electrolyte composition comprises: a) a polymer network with polyether backbone (component I); b) a low molecular weight linear, branched, hyper-branched polymer or any binary combination of such polymers with preferably non-reactive end groups (component-ll and / or component-Ill, for formation of ternary semi-IPN system); c) an electrolyte salt and / or a redox pair, and optionally d) a bare or surface modified nanostructured material to form a nanocomposite.
Owner:COUNCIL OF SCI & IND RES
Solid-state battery and method for manufacturing of such a solid-state battery
InactiveUS20100233548A1Lower impedanceImprove battery performanceFinal product manufactureElectrode carriers/collectorsSolid state electrolyteElectricity
Batteries based on solid-state electrolytes are known in the art. These (planar) energy sources, or solid-state batteries, efficiently convert chemical energy into electrical energy and can be used as the power sources for portable electronics. The invention relates to a method for manufacturing of a solid-state battery in which the pinholes in a solid electrolyte are at least partially filled by the deposition of an electrically insulating layers. The invention also relates to a battery obtained by performing such a method. The invention further relates to an electronic device provided with such a battery.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
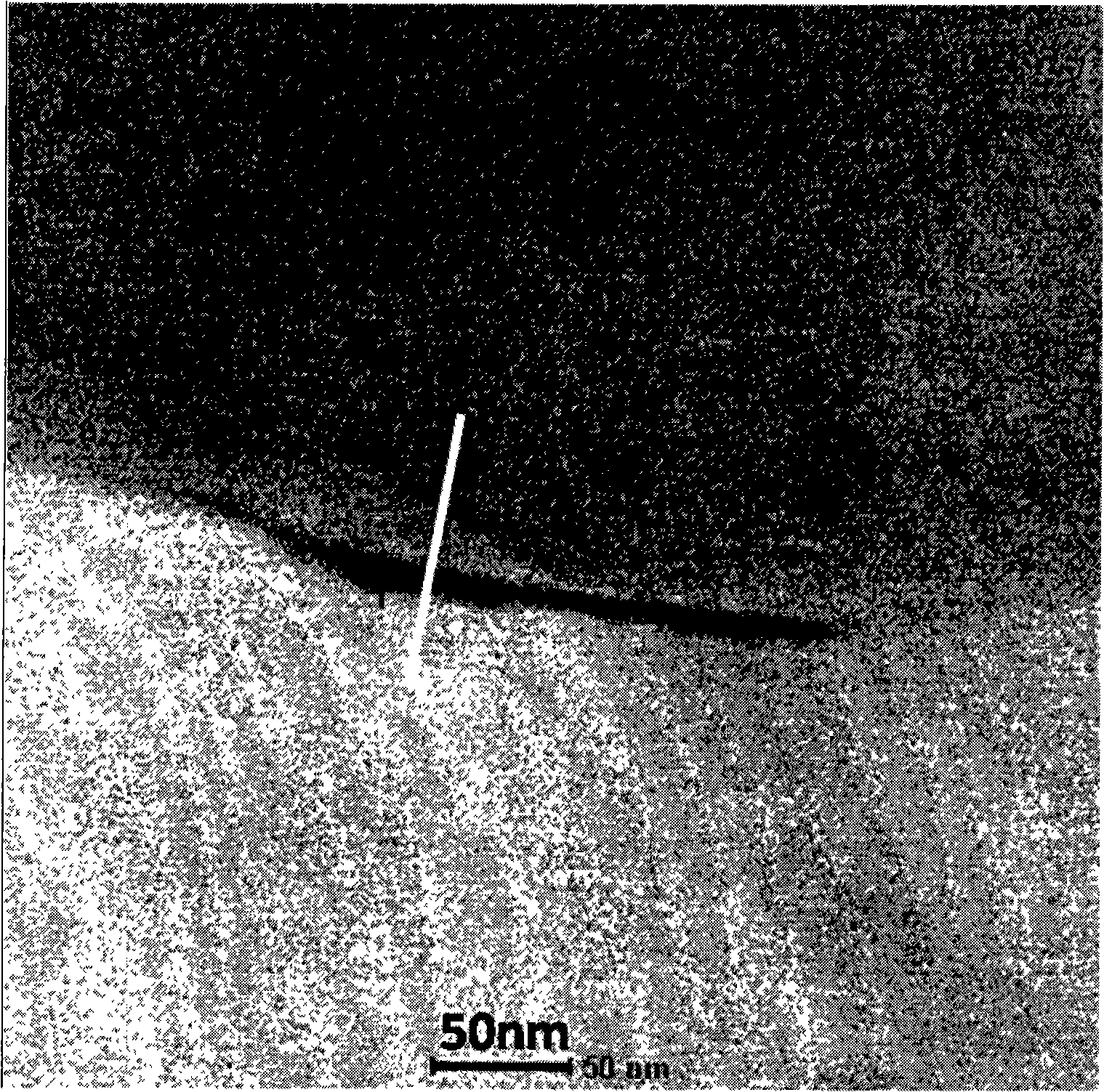
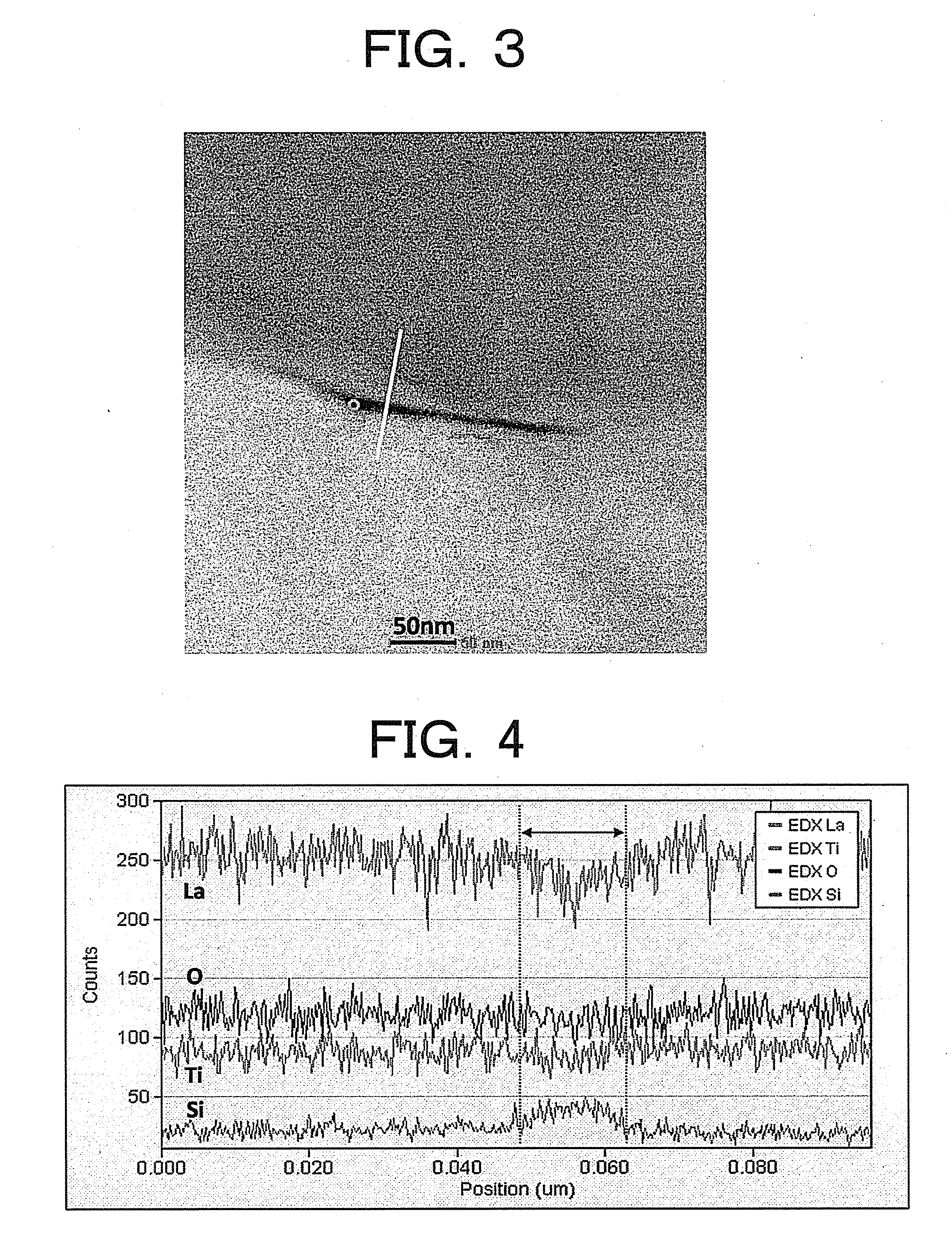
Lithium lanthanum titanium oxygen LLTO composite solid-state electrolyte material and synthesizing method thereof
ActiveCN101325094AGood lifting effectSimple processSolid electrolyte cellsSecondary cellsSolid state electrolyteComposite ceramic
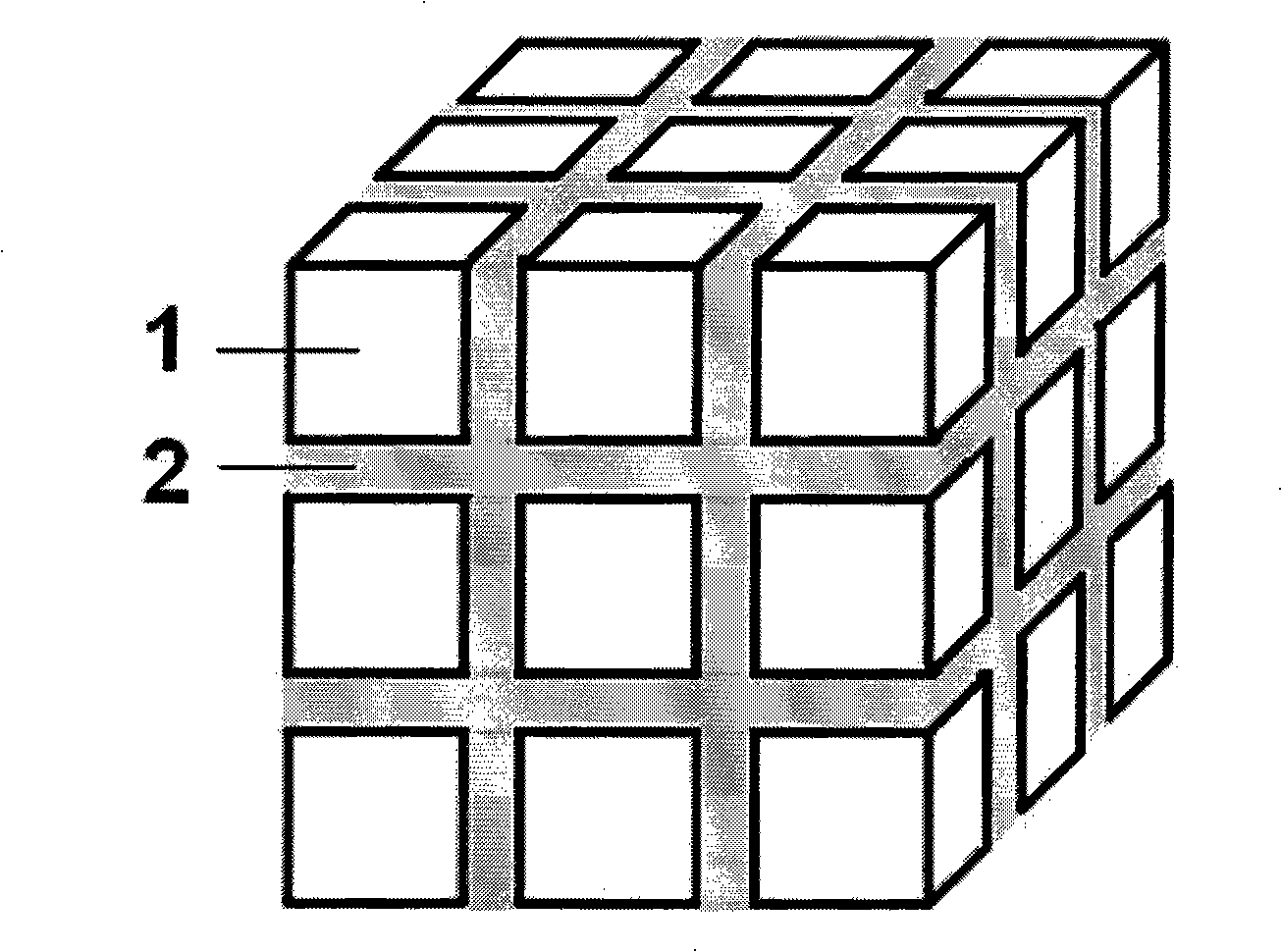
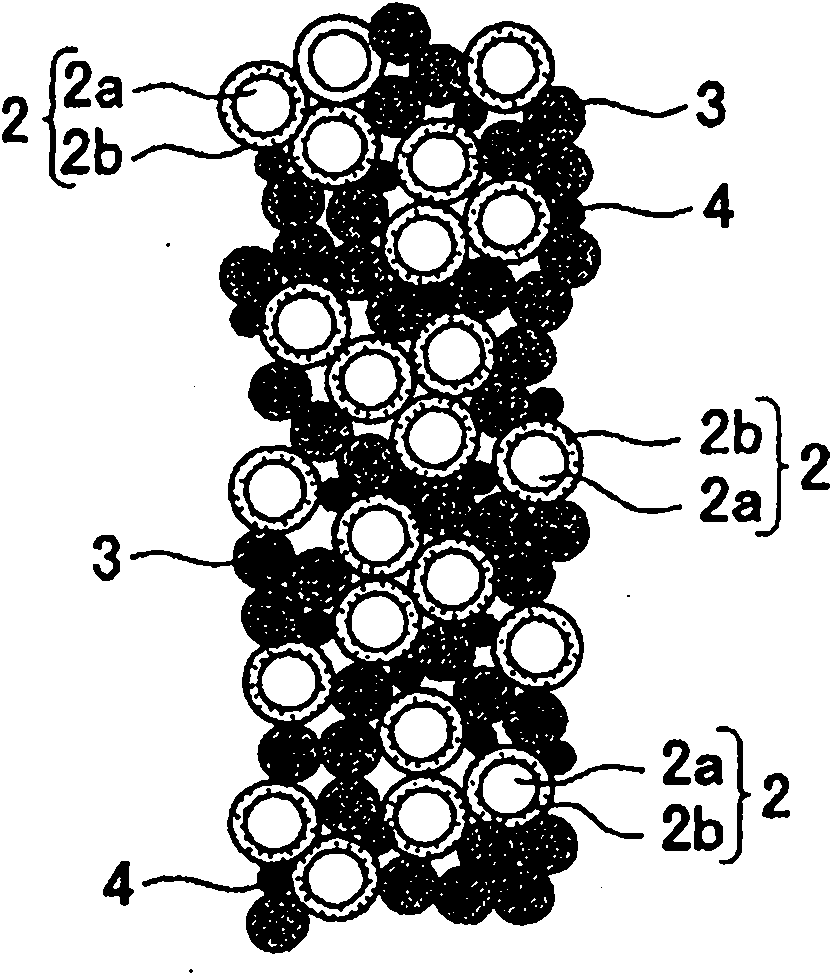
The invention provides a La-Li-Ti-O (LLTO) composite solid electrolyte material containing an amorphous silicon oxidate grain boundary layer and the synthetic method thereof, and belongs to the field of the lithium ion battery. The material is characterized in that: composite ceramics of an amorphous nano-silicon oxidate layer 2 are contained in the position of the grain boundary between materialcrystal grains, and the induction of the amorphous nano-silicon oxidate layer 2 is realized by adopting the wet chemical process, in the wet chemical process, inexpensive organic silicide is adopted as the additive to be added to the LLTO solid electrolyte material, and when the silicone content is 1 to 10 percent, the LLTO composite solid electrolyte material containing the amorphous silicon oxidate grain boundary layer can be synthesized through agglomeration. The electrical conductivity of the grain boundary is obviously improved, thereby improving the total electrical conductivity of the material. The composite solid electrolyte material has the advantages that the preparation process is simple, the operation is easy, the experimental period is greatly shortened, and the synthesis temperature is reduced, the energy consumption and the production cost are saved.
Owner:TSINGHUA UNIV +1
Li-la-ti-o composite solid electrolyte material containing silicon and synthesizing method thereof
ActiveUS20110059369A1Easy to implementImprove conductivitySecondary cellsSolid electrolyte cellsExperimental methodsAmorphous silicon
The invention relates to a lithium lanthanum titanate composite solid electrolyte material containing silicon in which amorphous Si or an amorphous Si compound exist in a grain boundary between crystal grains, and a method of producing the same, and belongs to a field of a lithium ion battery. According to the invention, the amorphous Si or the amorphous Si compound exist in the grain boundary between the crystal grains of the lithium lanthanum titanate. The amorphous Si or the amorphous Si compound are introduced into the grain boundary by employing a wet chemical method. In the wet chemical method, the inexpensive organosilicon compound is used as an additive, and the organosilicon compound is added into the lithium lanthanum titanate solid electrolyte material. Thus, it is possible to synthesize the lithium lanthanum titanate composite solid electrolyte material containing silicon by performing sintering when the ratio of mass of the Si or mass of the Si calculated based on mass of the Si compound to mass of the lithium lanthanum titanate is 0.27% to 1.35%. Grain boundary conductivity thereof is significantly improved, and therefore, total conductivity is improved. In addition, processes of the experimental method are simple and easily performed. Also, an experimental period is greatly reduced, a synthesis temperature is reduced, and energy consumption and production cost are reduced.
Owner:TOYOTA JIDOSHA KK +1
Ionically-conductive reinforced glass ceramic separators/solid electrolytes
ActiveUS20180375148A1Increased durabilityImprove fracture toughnessSolid electrolytesLi-accumulatorsThermoplasticPolymer science
Fiber-reinforced separators / solid electrolytes suitable for use in a cell employing an anode comprising an alkali metal are disclosed. Such fiber-reinforced separators / solid electrolytes may be at least partially amorphous and prepared by compacting, at elevated temperatures, powders of an ion-conducting composition appropriate to the anode alkali metal. The separators / solid electrolytes may employ discrete high aspect ratio fibers and fiber mats or plate-like mineral particles to reinforce the separator solid electrolyte. The reinforcing fibers may be inorganic, such as silica-based glass, or organic, such as a thermoplastic. In the case of thermoplastic fiber-reinforced separators / solid electrolytes, any of a wide range of thermoplastic compositions may be selected provided the glass transition temperature of the polymer reinforcement composition is selected to be higher than the glass transition temperature of the amorphous portion of the separator / solid electrolyte.
Owner:GM GLOBAL TECH OPERATIONS LLC
Composite membrane, preparation method of composite membrane, and lithium-ion battery
InactiveCN103474601AEvenly dispersedImprove electrochemical cycle performanceSecondary cellsCell component detailsPolyolefinHexafluoropropylene
The invention relates to a method for preparing a composite membrane. The method comprises the following steps: a solution with nano sol is prepared, and the nano sol is selected from at least one of titanium sol, alumina sol, silica sol and zirconium sol; a silane coupling agent and methyl methacrylate are added to the solution; the solution is uniformly mixed to form a first mixture; an initiator is added to the mixture to polymerize the methyl methacrylate and the silane coupling agent while the silane coupling agent and the nano sol-gel generates condensation reaction, therefore the nano sol is grafted on a polymethyl methacrylate substrate to form an inorganic-organic grafted hybrid polymer; the inorganic-organic grafted hybrid polymer is mixed with a copolymer of vinylidene fluoride and hexafluoropropylene in an organic solvent to form a uniform second mixture; the second mixture is spread onto the surface of a porous polyolefin membrane; the porous polyolefin membrane coated with the second mixture is dried. The invention further relates to the composite membrane and a lithium-ion battery.
Owner:JIANGSU HUADONG INST OF LI ION BATTERY +1
Solid-state hybrid electrolytes, methods of making same, and uses thereof
Provided are solid-state hybrid electrolytes. The hybrid electrolytes have a polymeric material layer, which may be a polymer / copolymer layer or a gel polymer / copolymer layer, disposed on at least a portion of an exterior surface or all of the exterior surfaces of a solid-state electrolyte. A hybrid electrolyte can form an interface with an electrode of an ion-conducting battery that exhibits desirable properties. The solid-state electrolyte can comprise a monolithic SSE body, a mesoporous SSE body, or an inorganic SSE having fibers or strands, which may be aligned. In the case of solid-state electrolytes that have strands, the strands can be formed using a sacrificial template. The hybrid solid-state electrolytes can be used in ion-conducting batteries, which may be flexible, ion-conducting batteries.
Owner:UNIV OF MARYLAND
Composite solid-state electrolyte, solid-state battery and preparation method of solid-state battery
InactiveCN111244537AImprove conductivityImprove mechanical propertiesSecondary cellsComposite electrolytesSolid state electrolyteElectrical battery
The invention belongs to the technical field of batteries, and particularly relates to a composite solid-state electrolyte which comprises an organic polymer, a lithium salt, an ionic liquid and an inorganic solid electrolyte material, wherein the mass ratio of the polymer to the lithium salt to the ionic liquid to the inorganic solid electrolyte material is 1: (0.1-0.6): (0.1-1): (0.05-0.2). Thecomposite solid-state electrolyte disclosed by the invention is high in ionic conductivity, high in stability, good in compatibility with positive and negative electrodes and excellent in mechanical property, and the composite solid-state electrolyte has excellent mechanical property due to a polymer framework; the lithium salt provides lithium ions for the solid electrolyte; the ionic liquid improves the conductivity of the electrolyte; the inorganic solid electrolyte material can be crosslinked with the polymer to further improve the mechanical properties of the solid electrolyte and increase the strength.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
Stable proton exchange membranes and membrane electrode assemblies
A proton exchange membrane and a membrane electrode assembly for an electrochemical cell such as a fuel cell are provided. A catalytically active component is disposed within the membrane electrode assembly. The catalytically active component comprises particles containing a metal oxide such as silica, metal or metalloid ions such as ions that include boron, and a catalyst. A process for increasing peroxide radical resistance in a membrane electrode is also provided that includes the introduction of the catalytically active component described into a membrane electrode assembly.
Owner:THE CHEMOURS CO FC LLC
Polymer electrolyte membrane for a fuel cell, and method for preparing same
ActiveUS20120231355A1Improve heat resistanceImprove proton conductivityMaterial nanotechnologyElectrolyte holding meansPolymer electrolytesFuel cells
The present disclosure relates to a polymer electrolyte membrane having a construction wherein an ionomer is charged in pores of a nanoweb having a high melting point, being insoluble in an organic solvent and having excellent pore characteristics, under optimum conditions. Therefore, an overall thickness of the electrolyte membrane may be reduced, thereby attaining advantages such as decrease in ohmic loss, reduction of material costs, excellent heat resistance, low thickness expansion rate which in turn prevents proton conductivity from being deteriorated over a long term. The polymer electrolyte membrane of the present invention comprises a porous nanoweb having a melting point of 300□ or more and being insoluble in an organic solvent of NMP, DMF, DMA, or DMSO at room temperature; and an ionomer which is charged in pores of the porous nanoweb and contains a hydrocarbon material soluble in the organic solvent at room temperature.
Owner:KOLON IND INC
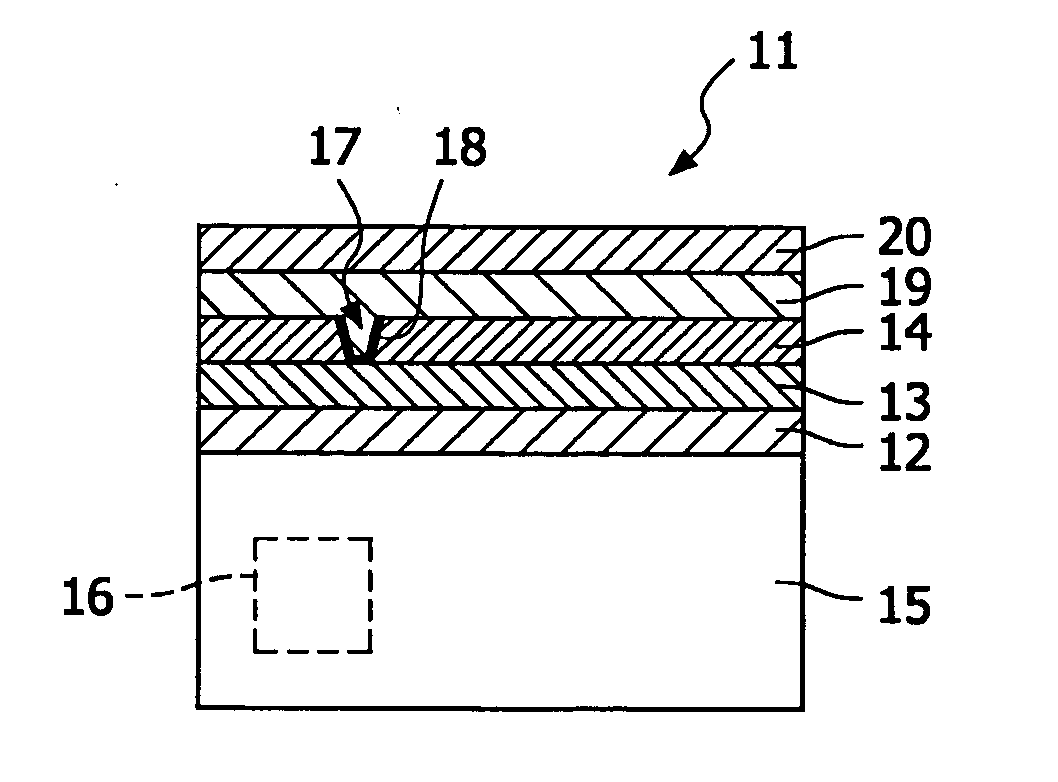
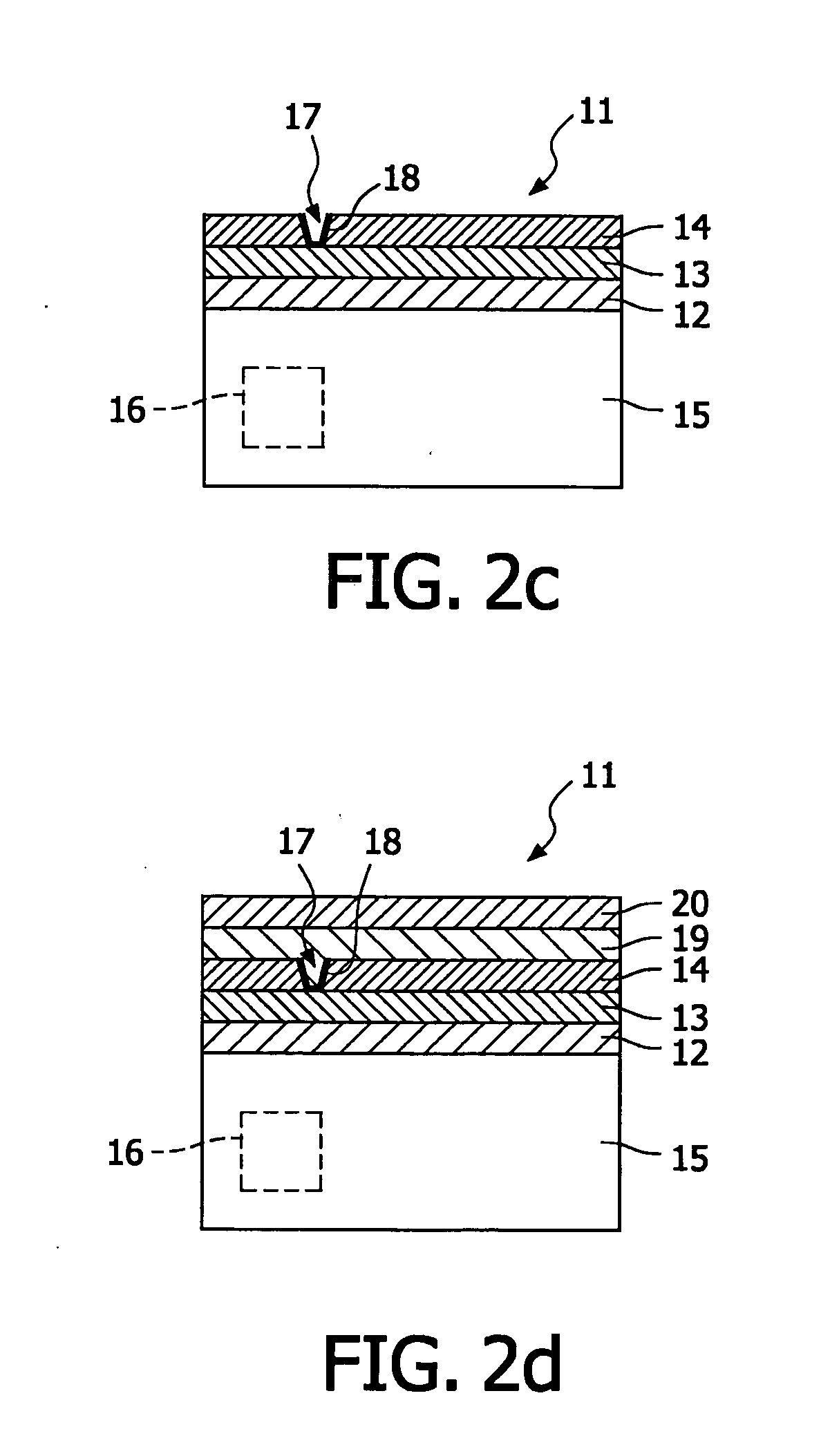
Electrolyte membrane and membrane electrode assembly using the same
InactiveUS20100143823A1Relieve pressureIncreased durabilityElectrolyte holding meansSolid electrolytesPolymer electrolytesSwelling ratio
An electrolyte membrane (11) includes: a filler (20); and a polymer electrolyte (22). A thickness of the electrolyte membrane (11) is 1 micrometer to 500 micrometer, a moisture content thereof is 10 mass % or more, and a ratio of a swelling ratio in a membrane surface direction (xy) thereof and a swelling ratio in a membrane thickness direction (z) thereof satisfies following Expression 1: where Lambda z is the swelling ratio in the membrane thickness direction (z), and Lambda xy is the swelling ratio in the membrane surface direction (xy).λxyλz<0.3[Math.1]
Owner:NISSAN MOTOR CO LTD
Composite solid electrolyte material and preparation method and application thereof
ActiveCN110085909AGood compatibilityImprove electrochemical performanceSolid electrolytesSecondary cellsAll solid statePolymer electrolytes
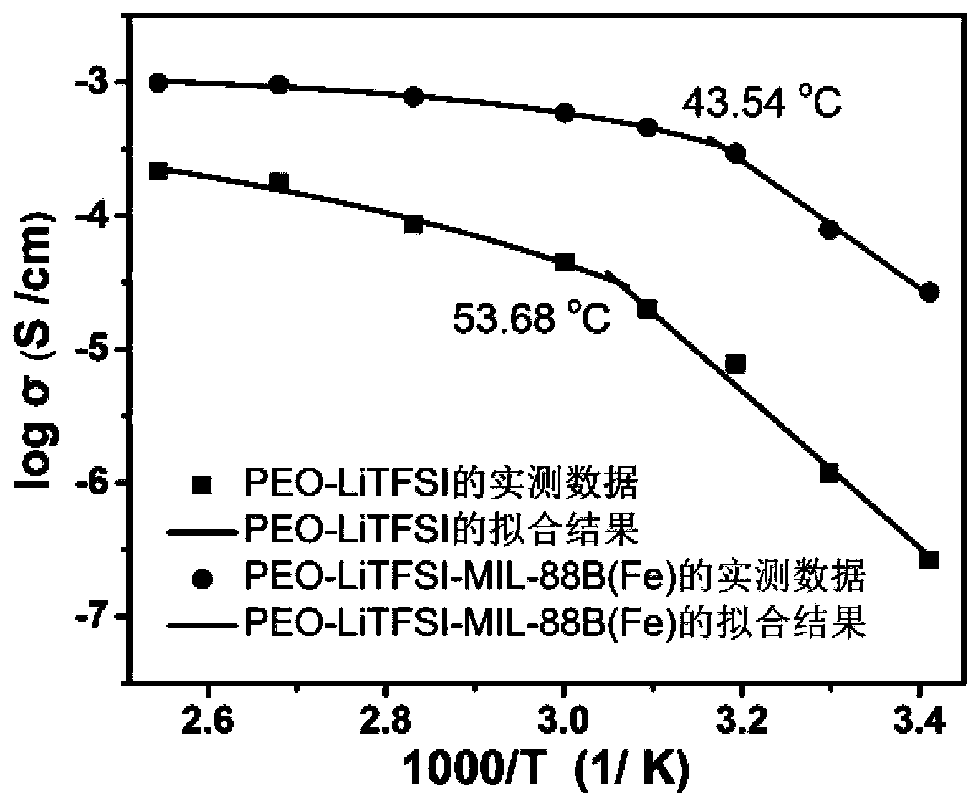
The invention discloses a composite solid electrolyte material and a preparation method and application thereof. The composite solid electrolyte material is composed of a conducting ion polymer, a metal-organic framework material and an alkali metal or alkaline earth metal salt. The metal-organic framework material includes MOF-235, MIL-68, MIL-88. MIL-96 and other series. The metal-organic framework material has a special topological structure. The addition of the solid electrolyte material can effectively reduce the crystallinity of the polymer electrolyte, promote the dissociation of the alkali metal or alkaline earth metal salt, the obtained composite solid electrolyte has good ionic conductivity and electrochemical stability in a wide temperature range (25-120 DEG C) and also has goodflexibility and film thinning, and the preparation method is simple and scale production is feasible. The composite solid electrolyte can be matched with different types of positive electrode materials and alkali metal or alkaline earth metal negative electrode, and the assembled all-solid-state battery can present good electrochemical performance at the above-mentioned temperature.
Owner:CENT SOUTH UNIV
Electrode element, method of manufacturing electrode element, and lithium ion secondary battery
InactiveCN101953000AImprove performanceSolid electrolytesNon-aqueous electrolyte accumulator electrodesLithiumEngineering
An electrode element contains a positive electrode active material and a second solid electrolyte. The positive electrode active material has an active material and a first solid electrolyte. Seventy percent or more of a surface of the active material is coated with the first solid electrolyte.
Owner:TOYOTA JIDOSHA KK +1
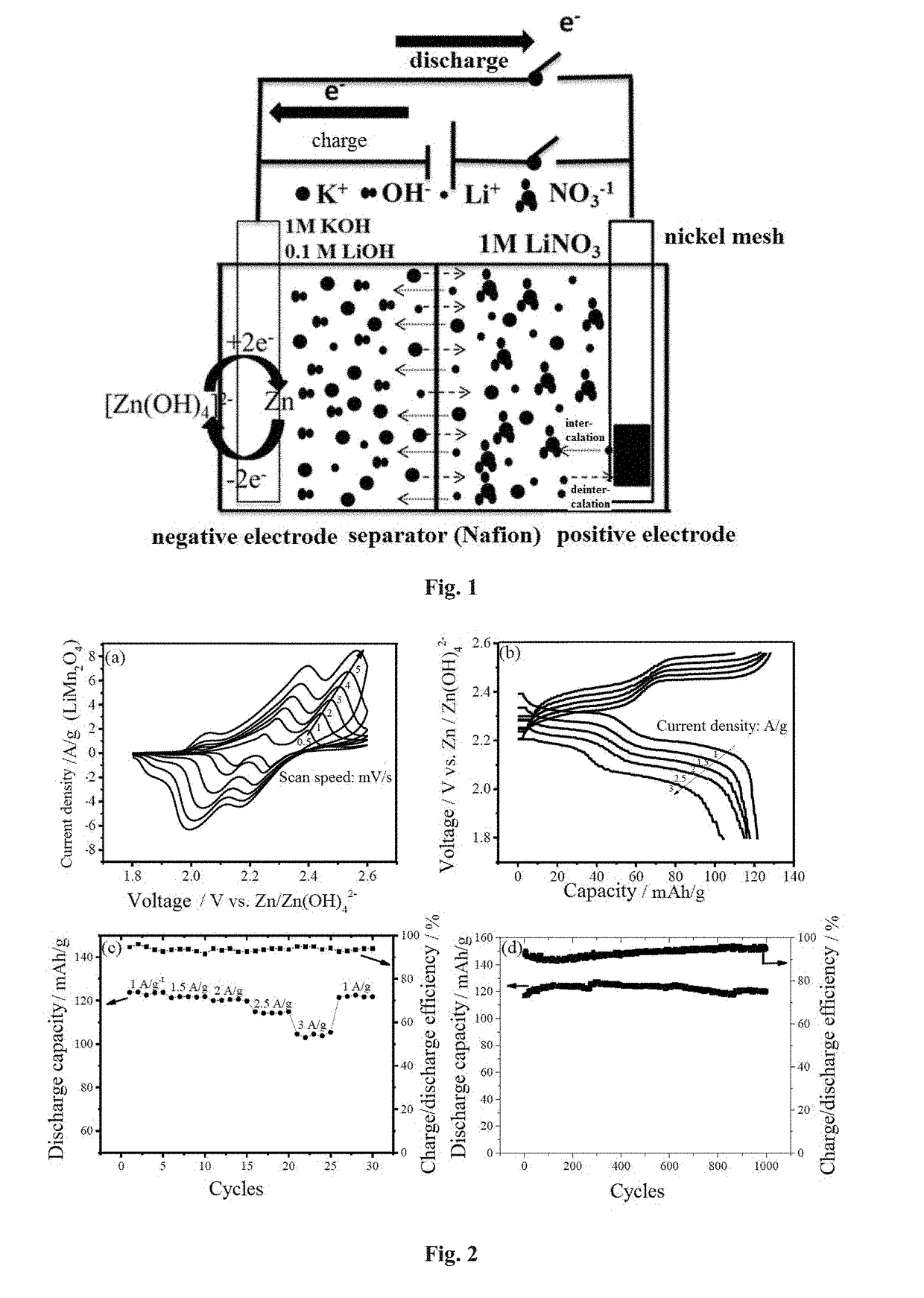
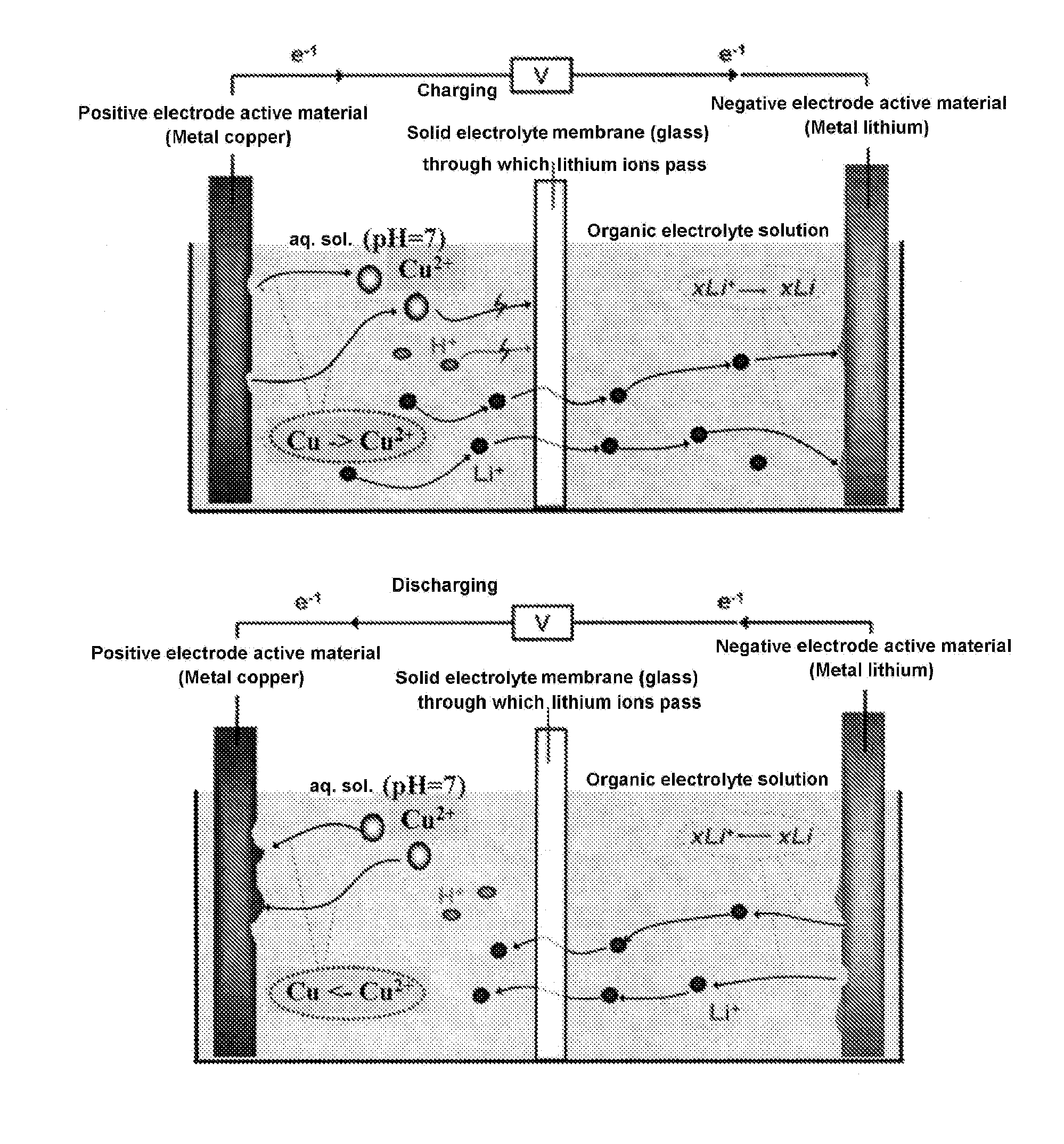
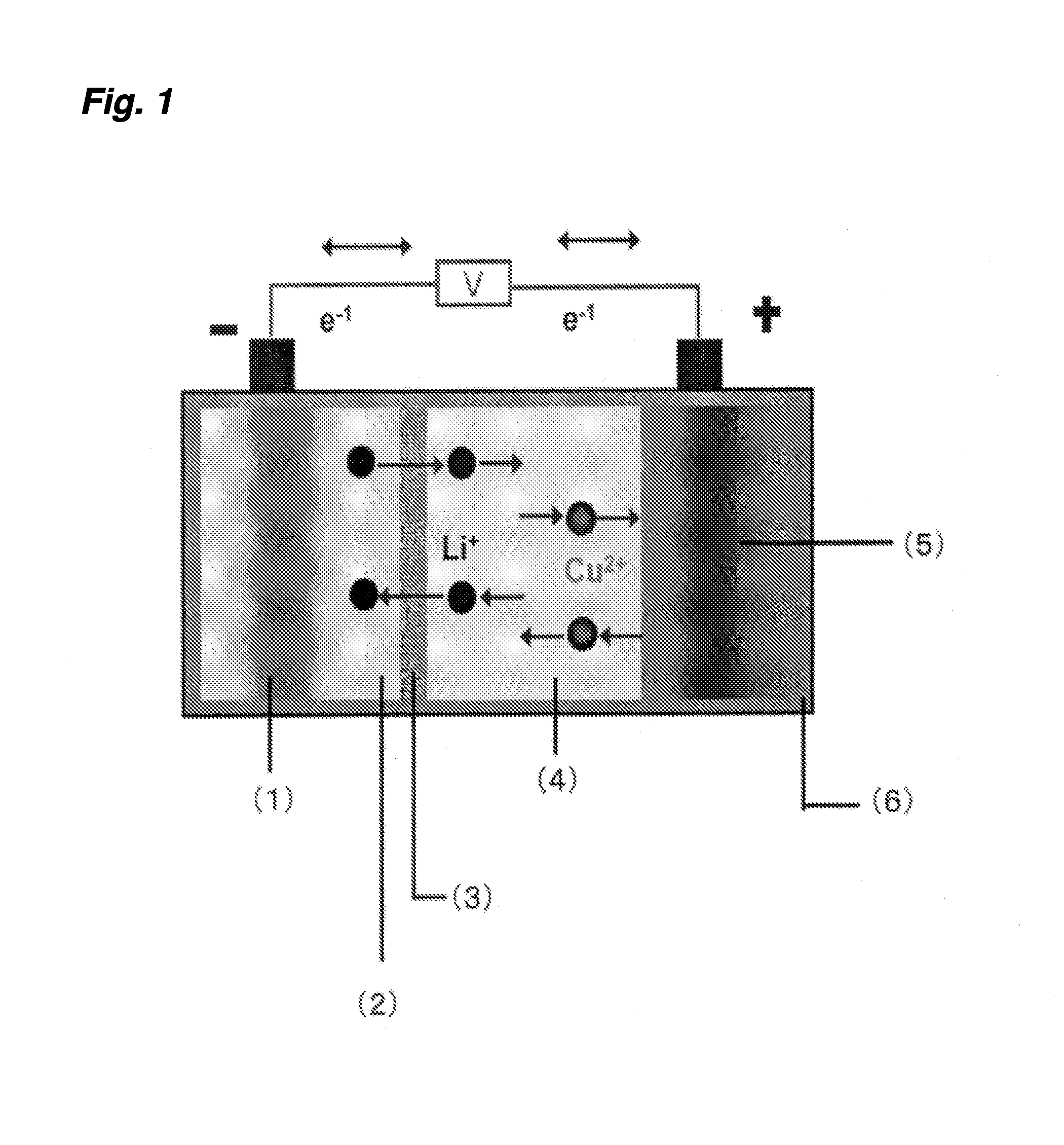
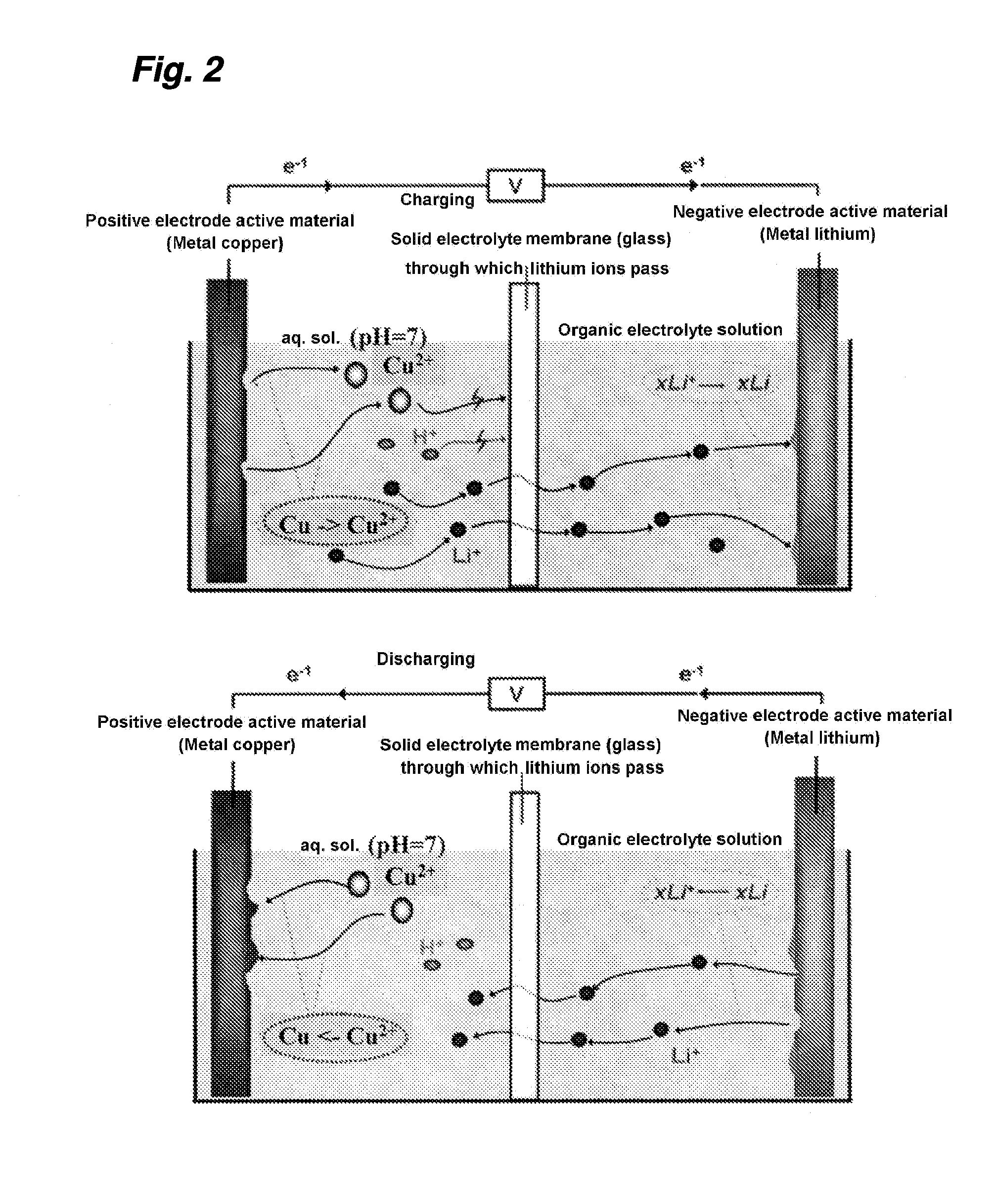
Hybrid aqueous rechargeable battery
InactiveUS20190036147A1Improve stabilityImprove cycle performanceSolid electrolytesFuel and secondary cellsElectricityElectrical battery
The disclosure relates to a hybrid aqueous rechargeable battery, wherein the battery comprises a positive electrode and a negative electrode, and wherein the positive electrode and the negative electrode are in aqueous electrolytes with different pHs respectively. The hybrid aqueous rechargeable battery has a working voltage at least 0.6 V higher than the conventional aqueous lithium batteries and a theoretical energy density at least 60 Wh / kg higher. The hybrid aqueous rechargeable battery can be used for storage and discharge of electricity, etc.
Owner:NANJING UNIV OF TECH
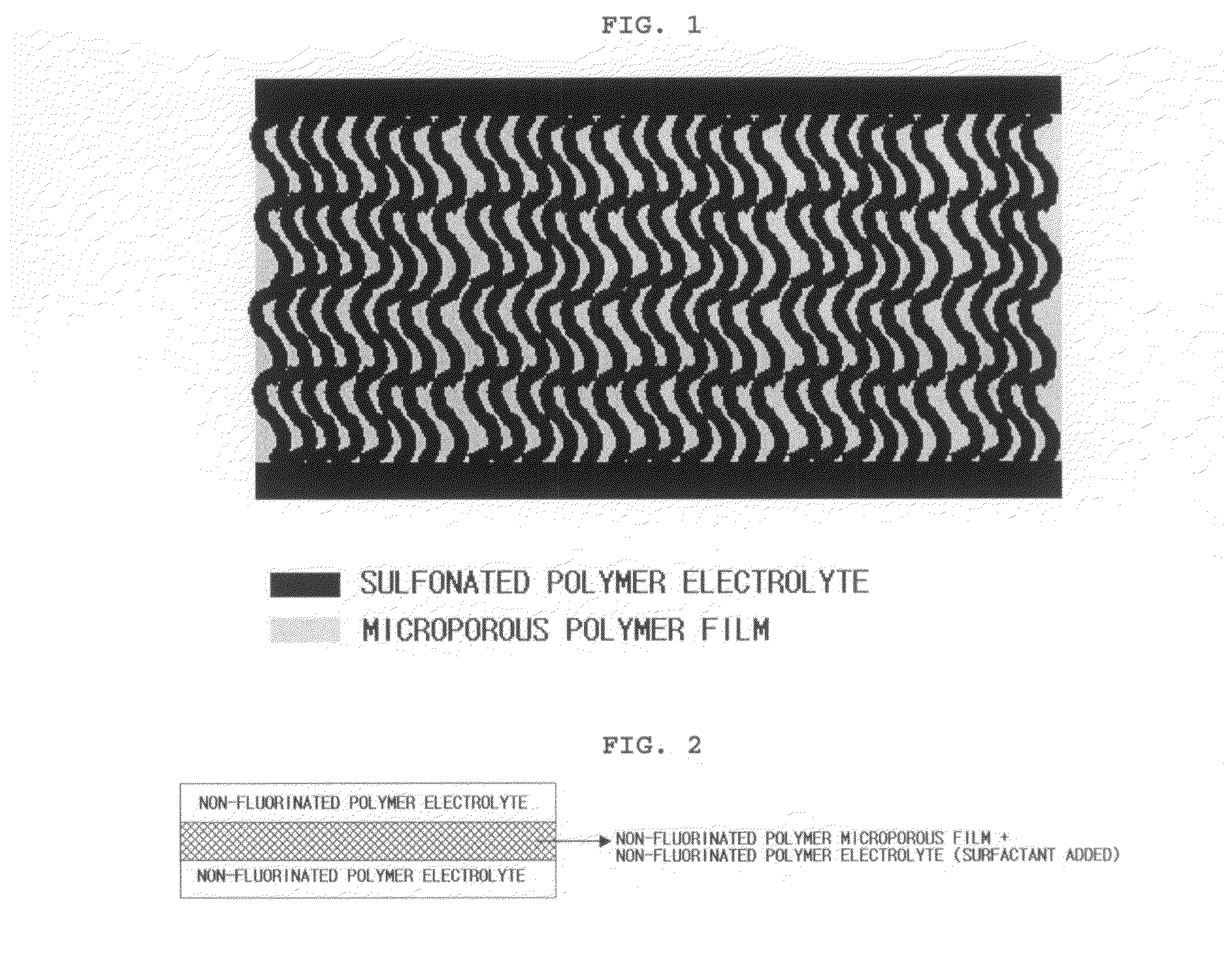
Reinforced composite electrolyte membrane for fuel cell
ActiveUS20070231653A1Quality improvementIncreased durabilitySolid electrolytesFinal product manufacturePolymer electrolytesPolymer science
Disclosed is a composite electrolyte membrane comprising a microporous polymer substrate and a sulfonated polymer electrolyte. The composite electrolyte membrane comprises: a first polymer electrolyte layer formed of a first non-fluorinated or partially-fluorinated sulfonated polymer electrolyte; a non-fluorinated or partially-fluorinated microporous polymer substrate stacked on the first polymer electrolyte layer, wherein pores of the microporous polymer substrate are impregnated with a second non-fluorinated or partially-fluorinated sulfonated polymer electrolyte, and the first polymer electrolyte and the second polymer electrolyte are entangled with each other on an interface thereof; and a third polymer electrolyte layer formed on the microporous polymer substrate impregnated with the second polymer electrolyte by a third non-fluorinated or partially-fluorinated sulfonated polymer electrolyte, wherein the second polymer electrolyte and the third polymer electrolyte are entangled with each other on an interface thereof. A method for manufacturing the composite electrolyte membrane, and a membrane-electrode assembly (MEA) and a fuel cell comprising the composite electrolyte membrane are also disclosed.
Owner:LG CHEM LTD
Composite ion exchange material
InactiveUS6902801B2Difficult to sulphonateEasy to sulphonateFinal product manufacturePretreated surfacesPolymer electrolytesFuel cells
A composite material, for example a composite membrane for a polymer electrolyte membrane fuel cell includes a first conductive polymer and a support material for the polymer, wherein the support material comprises a second conductive polymer. A method making of the composite material is also disclosed as is its use as a polymer electrolyte membrane in a fuel cell.
Owner:VICTREX MFG
Composite polymer electrolyte composition
Owner:PIOTREK
Composite solid polymer electrolyte membrane and preparation method and application thereof
InactiveCN111540948AIncrease energy densityImprove stabilityFinal product manufactureElectrolyte accumulators manufacturePolymer electrolytesPlasticizer
The invention discloses a composite solid polymer electrolyte membrane as well as a preparation method and application thereof. The composite solid polymer electrolyte membrane comprises a polymer component, a lithium salt, 0-25 parts by mass of a plasticizer, and 0-15 parts by mass of inorganic particles, wherein the polymer component comprises 10-30 parts by mass of a thermoplastic polymer and 20-50 parts by mass of a cross-linked network polymer, and the molar ratio of the lithium salt to lithium ion complexing dissociation groups in the polymer component is 1:(1-16). The composite solid polymer electrolyte membrane has high ionic conductivity and good mechanical strength, the preparation process is simple, and the composite solid polymer electrolyte membrane can be applied to large-scale production. In some embodiments of the present invention, the thickness of the composite solid polymer electrolyte membrane is 10-500 [mu]m, the tensile strength is 0.2 MPa or above, the lithium-ion conductivity is 1*10<-5> S / cm or above, and the electrochemical window is 3.8 V or above, so that the composite solid polymer electrolyte membrane can be used in the fields of solid lithium batteries, electrochromic devices and the like, and short circuit is avoided after long-time use at high temperature.
Owner:CHINA LUCKY FILM CORP
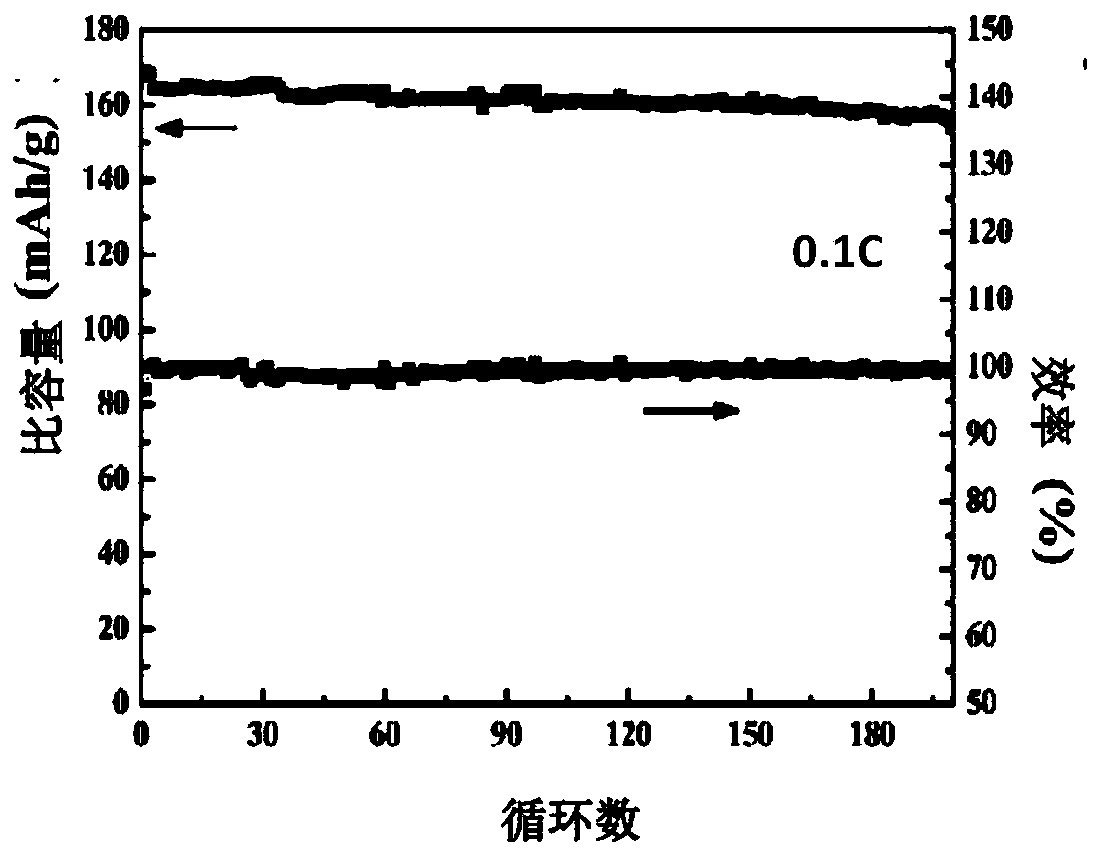
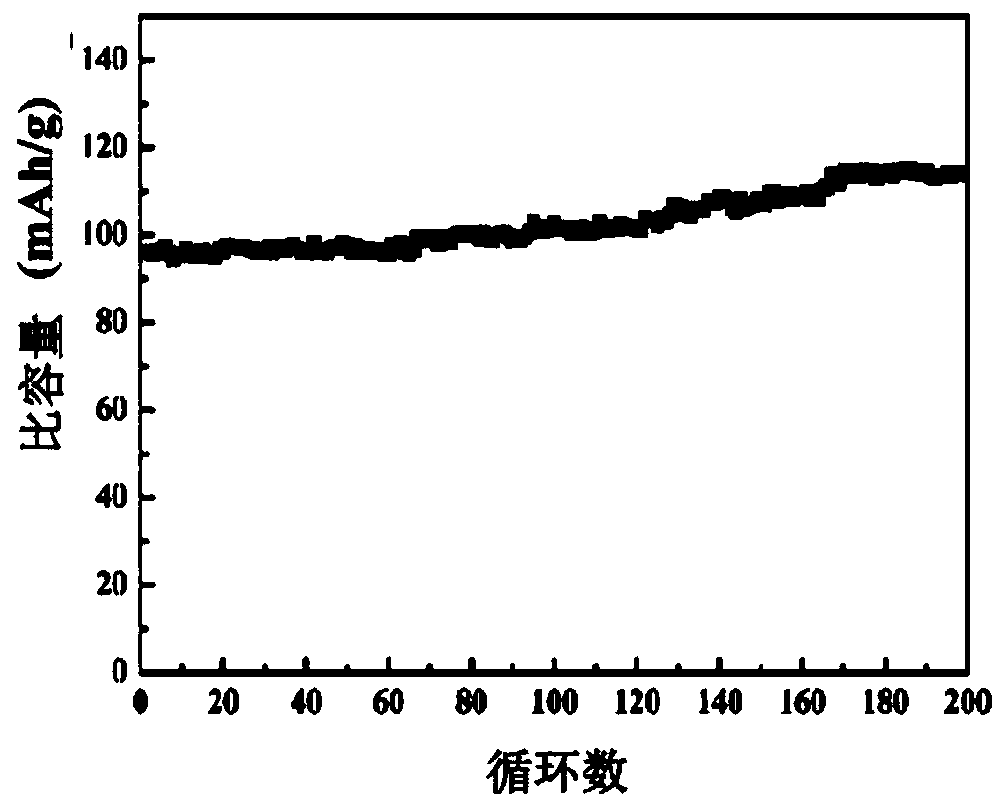
Polymerized organic-inorganic composite solid electrolyte and in-situ assembled all-solid-state battery
ActiveCN110556586AReduce interface resistanceImprove ionic conductivityFinal product manufactureElectrolyte accumulators manufactureCross-linkAll solid state
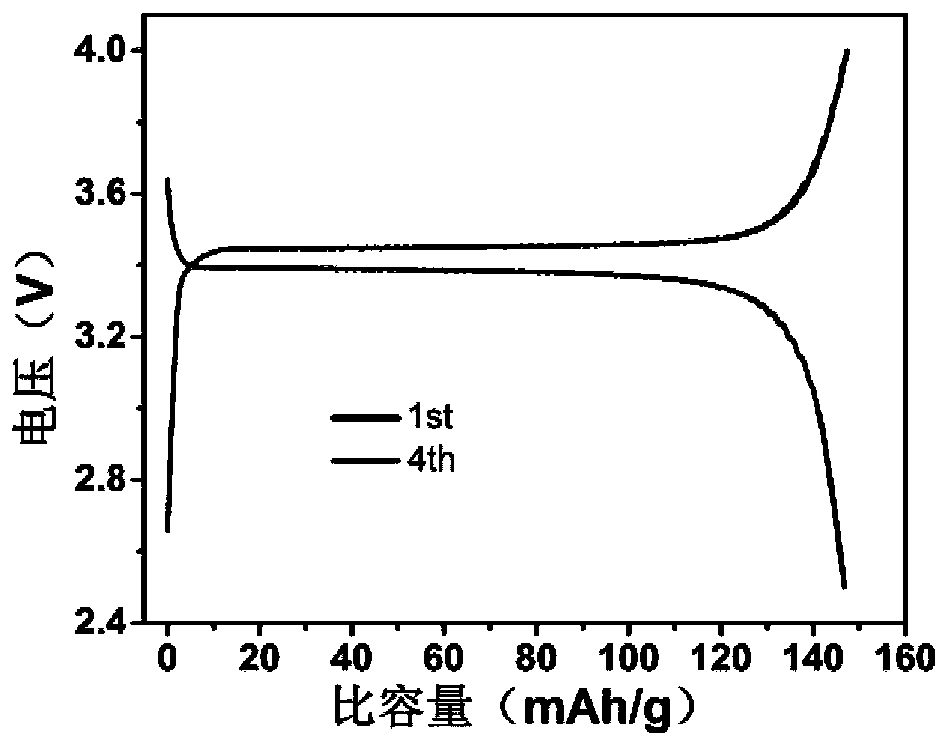
The invention relates to a polymerized organic-inorganic composite solid electrolyte and an in-situ assembled all-solid-state battery, and belongs to the technical field of ion battery preparation. The preparation method of the polymerized solid electrolyte comprises the following steps: fully and uniformly mixing a polymer monomer and a cross-linking agent, and adding an electrolyte salt and an initiator to obtain an electrolyte precursor; and initiating the electrolyte precursor to obtain the polymerized solid electrolyte. The in-situ assembled all-solid-state battery can be obtained by dropping the electrolyte precursor onto a positive electrode, covering the electrolyte precursor with a negative electrode, carrying out initiating and curing the electrolyte precursor. The room-temperature conductivity of the solid electrolyte reaches 1.6*10<-4>Scm<-1>, and the electrochemical window is greater than 6V. For the all-solid-state battery based on the solid electrolyte, the discharge capacity density is 145mAh / g at the charge-discharge rate of 0.5C, the discharge capacity is 176mAh / g at the charge-discharge rate of 0.1C, and the capacity retention ratio after 100 cycles at the charge-discharge rate of 0.5C is 88%.
Owner:HUAZHONG UNIV OF SCI & TECH
Polymer electrolyte as well as polymer electrolyte membrane, membrane electrode assembly and polymer electrolyte fuel cell using the same
InactiveUS20070134530A1Reduce pressureFilling of pore easyConductive materialSecondary cellsPolymer electrolytesFuel cells
The present invention relates to a polymer electrolyte that provides high proton conductivity and low fuel crossover at the same time, as well as a member using the same. The embodiments of the invention can achieve high output and high energy density in the form of a polymer electrolyte fuel cell. A polymer electrolyte comprising a proton conductive polymer (A) and a polymer (B) which is different from (A) wherein a ratio of the amount of unfreezable water, represented by formula (S1), in said polymer electrolyte is no less than 40 wt % and no greater than 100 wt % is disclosed. The ratio of amount of unfreezable water (S1)=(amount of unfreezable water) / (amount of low melting point water+amount of unfreezable water)×100 (%).
Owner:TORAY IND INC
Lithium secondary cell
InactiveUS20120208062A1Inhibit deteriorationIncrease capacitanceFinal product manufactureCell electrodesLithiumBattery cell
A lithium secondary cell, having: a negative electrode, a negative electrode-electrolyte solution, a separator, a positive electrode-electrolyte solution, and a positive electrode, which are disposed in this order, in which the separator is a solid electrolyte through which only lithium ions pass.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Composite electrolyte and electrochemical device using composite electrolyte and electronic device
ActiveCN110165295ASolve problems that exist in related fieldsSolid electrolytesSecondary cellsSolid state electrolyteComposite electrolyte
The invention relates to a composite electrolyte and an electrochemical device using the composite electrolyte and an electronic device, and particularly provides a composite electrolyte comprising aninorganic solid electrolyte; an organic solid electrolyte; an organic additive; and a lithium salt, wherein the organic additive has a boiling point within the range of 150-350 DEG C, and the contentof the lithium salt is within the range of about 12wt%-50wt% based on the total weight of the composite electrolyte. The composite electrolyte has good conductivity. The lithium ion battery preparedby the composite electrolyte can realize operation at room temperature and low temperature and has low battery impedance and high battery capacity.
Owner:NINGDE AMPEREX TECH
Aqueous zinc ion battery electrolyte and application thereof
PendingCN111900497AReduce solubilityImprove cycle lifeFinal product manufactureSecondary cells servicing/maintenanceElectrolytic agentHigh concentration
The invention discloses an aqueous zinc ion battery electrolyte and application thereof. The aqueous zinc ion battery electrolyte disclosed by the invention contains solvent water, high-concentrationelectrolyte salt and zinc salt;the zinc salt is a water-soluble salt; the high-concentration electrolyte salt is potassium bis(fluorosulfonyl) imide and / or potassium trifluoromethanesulfonate, and themass molar concentration is not less than 10mol / kg. According to the electrolyte, due to the fact that high-concentration potassium bis(fluorosulfonyl) imide and / or potassium trifluoromethanesulfonate are / is added, a large number of water molecules in the electrolyte can be consumed due to the strong solvation effect, the hydration effect of zinc ions is reduced, and zinc dendrites formed by thezinc ions in dissolution and deposition are inhibited; in addition, the strong solvation effect of the high-concentration electrolyte salt and solvent water molecules can reduce the electrochemical activity of the water molecules, improve the electrochemical window of the electrolyte, reduce the hydrogen evolution reaction, reduce the dissolution effect of zinc, inhibit the decomposition of the water molecules on the surface of the electrode, reduce the corrosion dissolution of the zinc negative electrode and prolong the cycle life of the aqueous zinc ion battery.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL
Composite polymer electrolyte composition
InactiveUS7732099B2Improve mechanical propertiesIncrease energy densityElectrolyte holding meansLight-sensitive devicesMonomer compositionElectrolysis
A totally solid polymer electrolyte composition with high ionic conductivity and enhanced mechanical properties is provided. This electrolyte composition is produced by polymerizing a monomer composition comprising a molten quaternary ammonium salt having a polymerizable functional group introduced therein and a charge transfer ion source in the presence of a polymeric reinforcing material. The polymeric reinforcing material can be formed into a composite of polymer blend morphology by dissolving the monomer composition and the reinforcing material in an appropriate organic solvent and polymerizing the solution. Alternatively, the composite can be obtained by impregnating a porous sheet or film as the reinforcing material with the monomer composition and effecting polymerization. An electrolyte for lithium ion battery can be obtained by selecting a lithium salt as the charge transfer ion source; an electrolyte for fuel cell by selecting a proton donor; and an electrolyte for dye sensitized solar cell by selecting a redox ion pair. A polymer electrolyte composition not containing the charge transfer ion source is also useful as an electrolyte for electrolytic capacitor.
Owner:PIOTREK
Composite polymer electrolyte composition
InactiveUS20060057465A1Improve mechanical propertiesIncrease energy densityElectrolyte holding meansLight-sensitive devicesMonomer compositionElectrolytic agent
A totally solid polymer electrolyte composition with high ionic conductivity and enhanced mechanical properties is provided. This electrolyte composition is produced by polymerizing a monomer composition comprising a molten quaternary ammonium salt having a polymerizable functional group introduced therein and a charge transfer ion source in the presence of a polymeric reinforcing material. The polymeric reinforcing material can be formed into a composite of polymer blend morphology by dissolving the monomer composition and the reinforcing material in an appropriate organic solvent and polymerizing the solution. Alternatively, the composite can be obtained by impregnating, a porous sheet or film as the reinforcing material with the monomer composition and effecting polymerization. An electrolyte for lithium ion battery can be obtained by selecting a lithium salt as the charge transfer ion source; an electrolyte for fuel cell by selecting a proton donor; and an electrolyte for dye sensitized solar cell by selecting a redox ion pair. A polymer electrolyte composition not containing the charge transfer ion source is also useful as an electrolyte for electrolytic capacitor.
Owner:PIOTREK
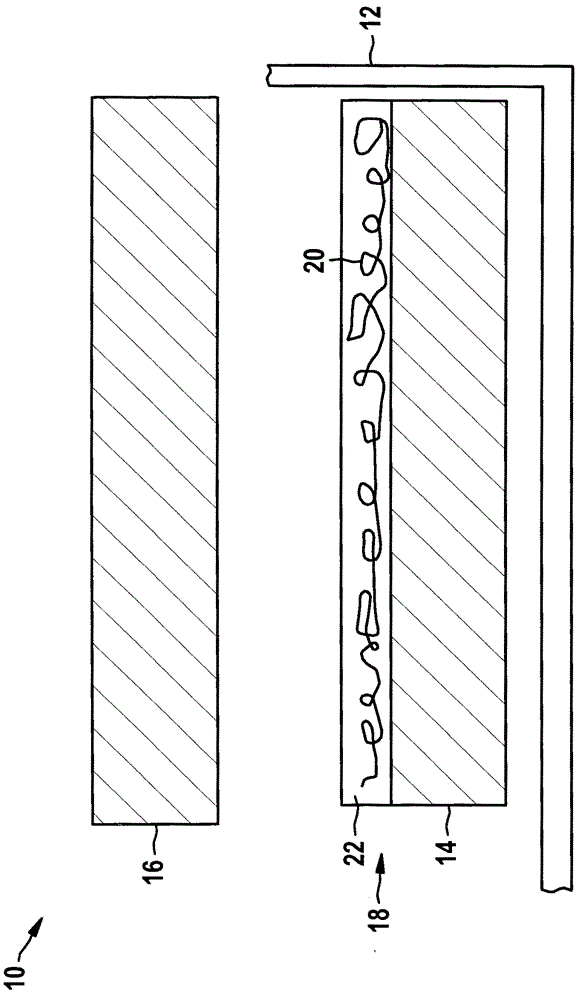
Electrochemical cell and method for the production thereof
Described is an electrochemical cell comprising a lithium-containing anode (14) and a cathode (16). The lithium-containing anode (14) has a protective layer (18) comprising fibers (20) which are made of a material not conducting lithium ions and which are in contact with a material (22) of the protective layer (18) that conducts lithium ions.
Owner:ROBERT BOSCH GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com