Solid electrolyte material manufacturable by polymer processing methods
a polymer processing and electrolyte technology, applied in the field of solid polymer electrolyte materials, can solve the problems of high polymer chain mobility, inability to develop an electrolyte, curtailment of polymer electrolyte adoption, etc., to improve li-based batteries, improve thermal and environmental stability, and improve energy density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
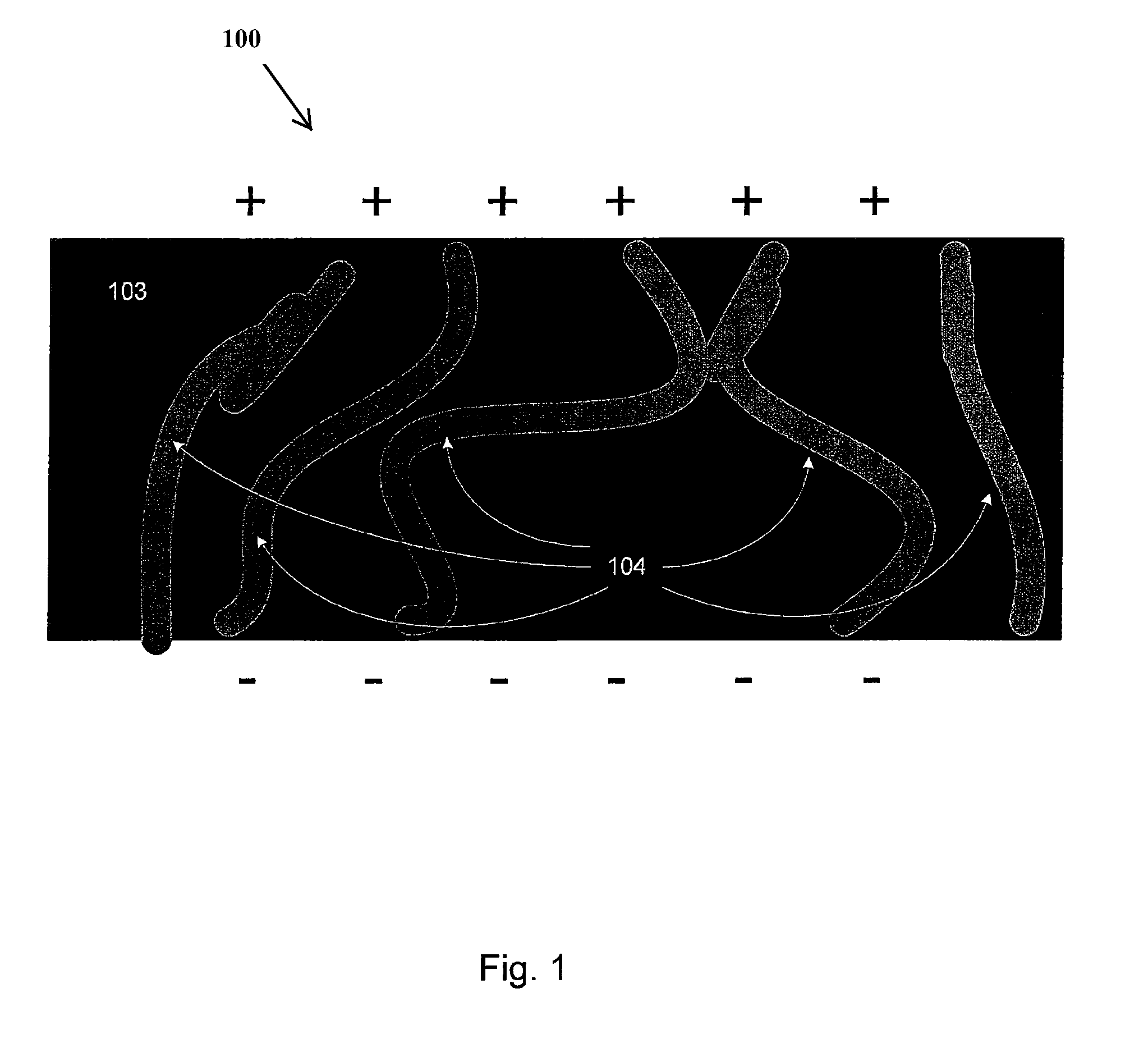
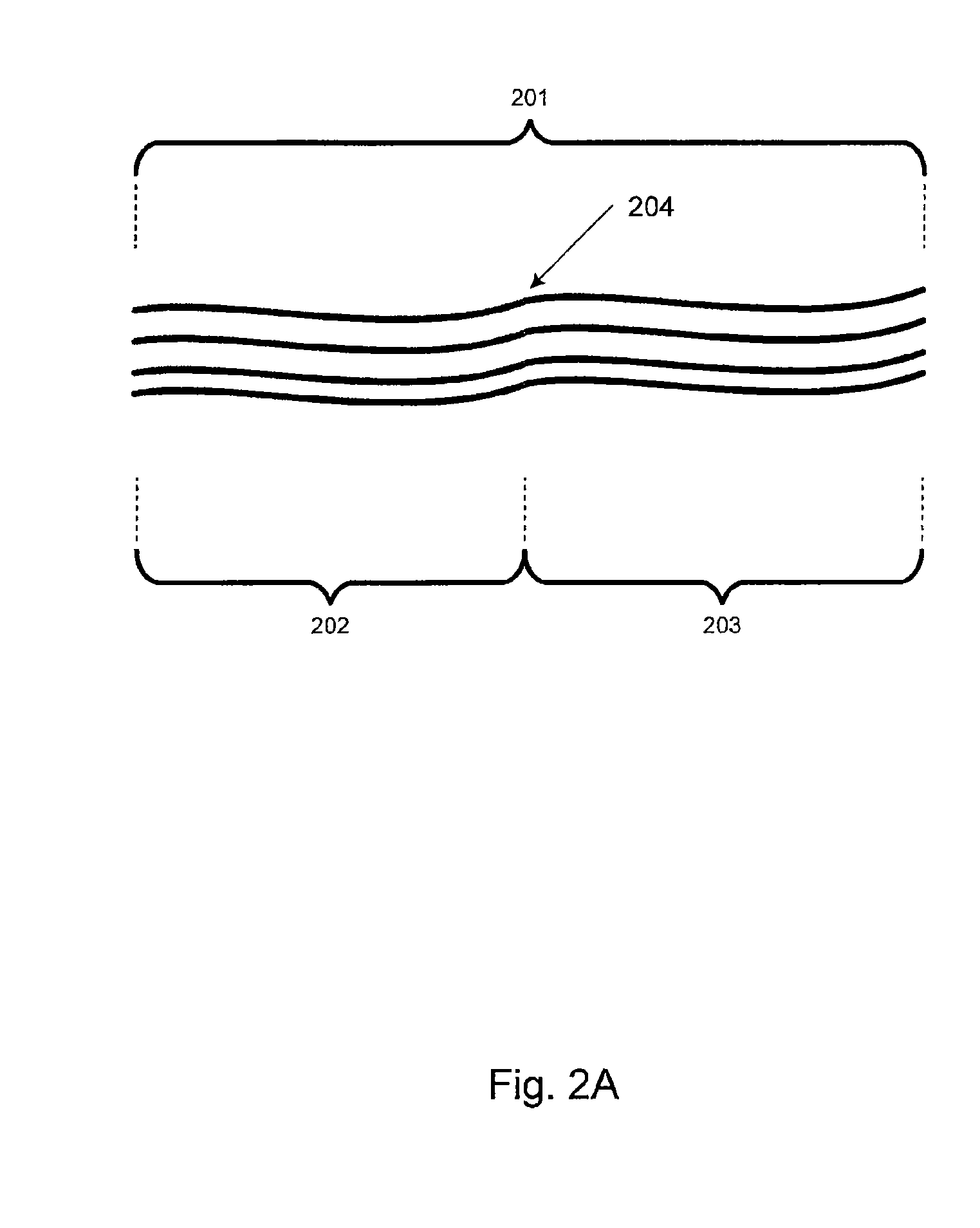
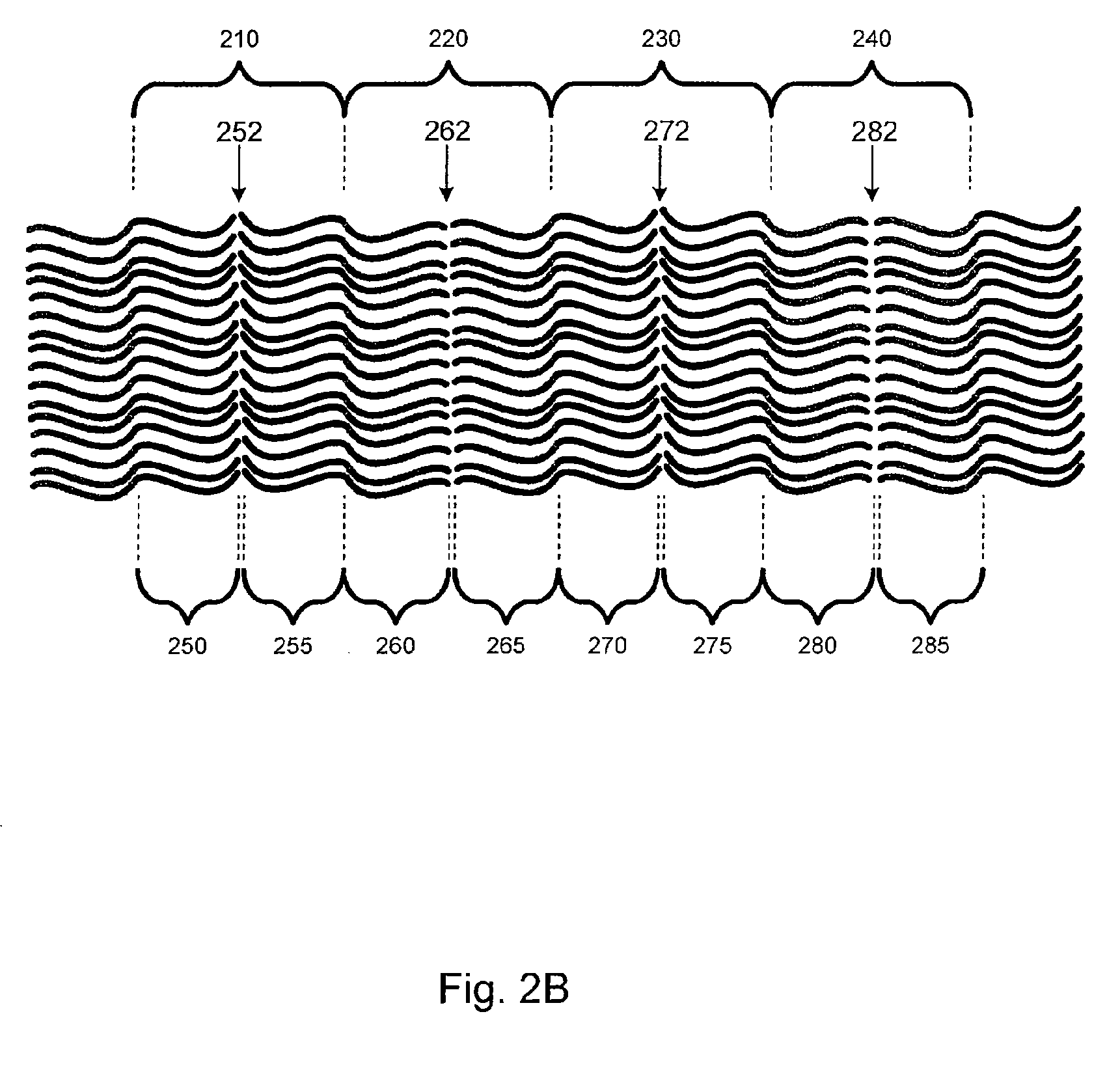
[0040]Embodiments of the present invention relate generally to electrolyte materials. More particularly, the embodiments relate to solid polymer electrolyte materials that are ionically conductive, mechanically robust, and manufacturable by conventional polymer processing methods. Merely by way of illustration, an exemplary polymer electrolyte material has an elastic modulus in excess of 1×106 Pa at 25 degrees C. and is characterized by an ionic conductivity of at least 1×10−5 Scm−1 at 90 degrees C. Many uses are contemplated for the solid polymer electrolyte materials. By way of example, the present invention can be applied to improve Li-based batteries by means of enabling higher energy density, better thermal and environmental stability, lower rates of self-discharge, enhanced safety, lower manufacturing costs, and novel form factors.
[0041]The current invention includes solid polymeric electrolyte materials. According to an embodiment, the material includes unique molecular archi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| elastic modulus | aaaaa | aaaaa |
| elastic modulus | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com