Polymerized organic-inorganic composite solid electrolyte and in-situ assembled all-solid-state battery
A solid electrolyte and all-solid-state battery technology, which is applied in the direction of composite electrolyte, electrolyte storage battery manufacturing, non-aqueous electrolyte storage battery, etc., can solve the problems of low conductivity and large interface resistance of all-solid-state batteries, and achieve reduced interface resistance and excellent battery performance , increase the effect of tightness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] (1) Preparation of the liquid precursor: mix the liquid polymer monomer and the crosslinking agent uniformly according to the predetermined ratio, then add the solid organolithium salt and the initiator, and stir until completely dissolved. Then add the ceramic electrolyte filler and stir until it is uniformly dispersed to obtain a completely mixed and uniform liquid precursor;
[0061] (2) Preparation of active ceramic electrolyte filler: Add corresponding salts to ethylene glycol in sequence, then add citric acid monohydrate and stir to obtain a clear solution; heat the obtained clear solution to reflux, age, carbonize, and then undergo high temperature Calcination yields active nanoparticles. In some embodiments, for the heat treatment of the carbide, the heat treatment temperature is set at 600-900°C, the heating rate is 5°C / min, and the carbonization time is 24h-48h. Active ceramic electrolyte fillers can also be formed by solid-state reactions, or nanofibrous fil...
Embodiment 1
[0067] Preparation of the precursor: mix the polymer monomer polyethylene glycol dimethacrylate and the crosslinking agent tetraethylene glycol diacrylate in a ratio of 19:1, then add 1mol / L LiTFSI and 2wt% heat Initiator azobisisobutyronitrile until completely dissolved. The subsequent addition of 10 wt% ceramic electrolyte Li 6.25 Ga 0.25 La 3 Zr 2 o 12 Filler, stir until evenly dispersed, and then a completely mixed and uniform liquid precursor can be obtained. The precursor can form a solid electrolyte under thermal initiation.
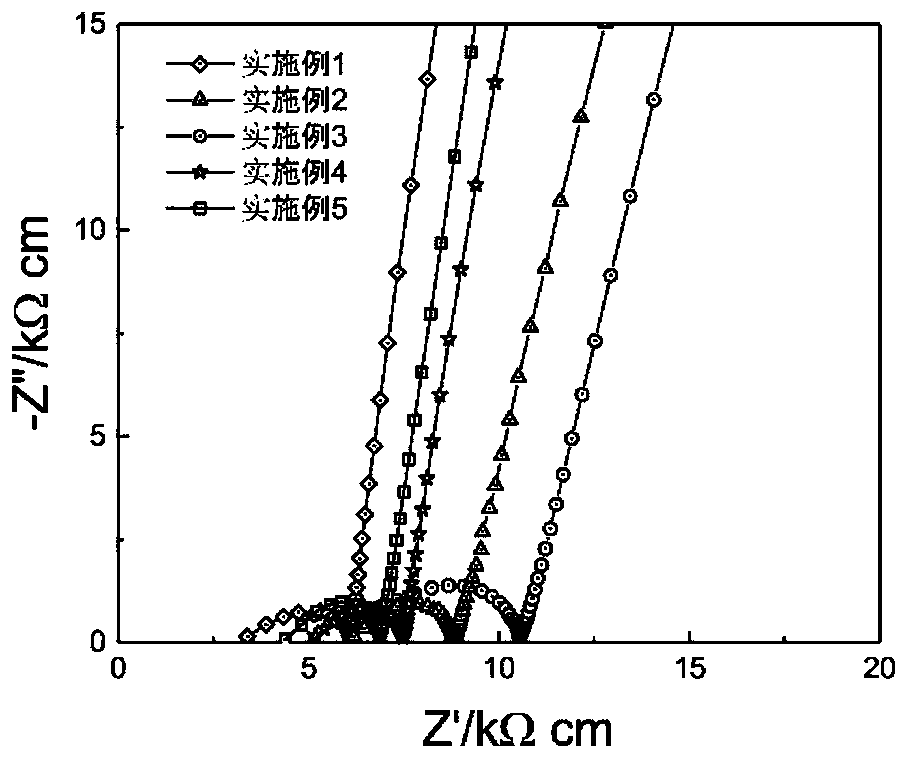
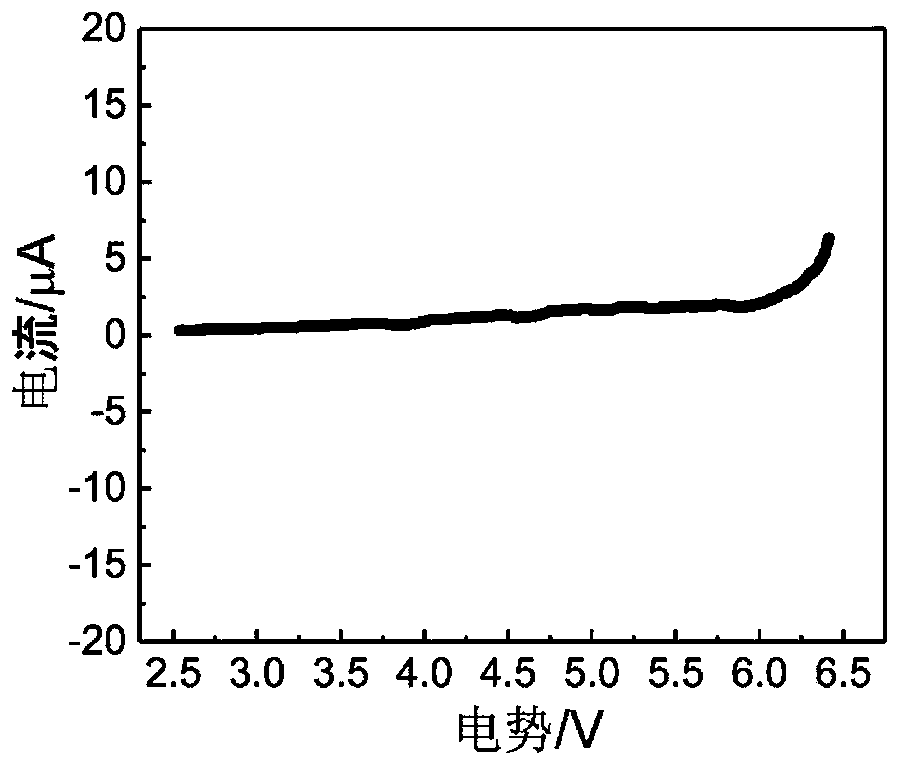
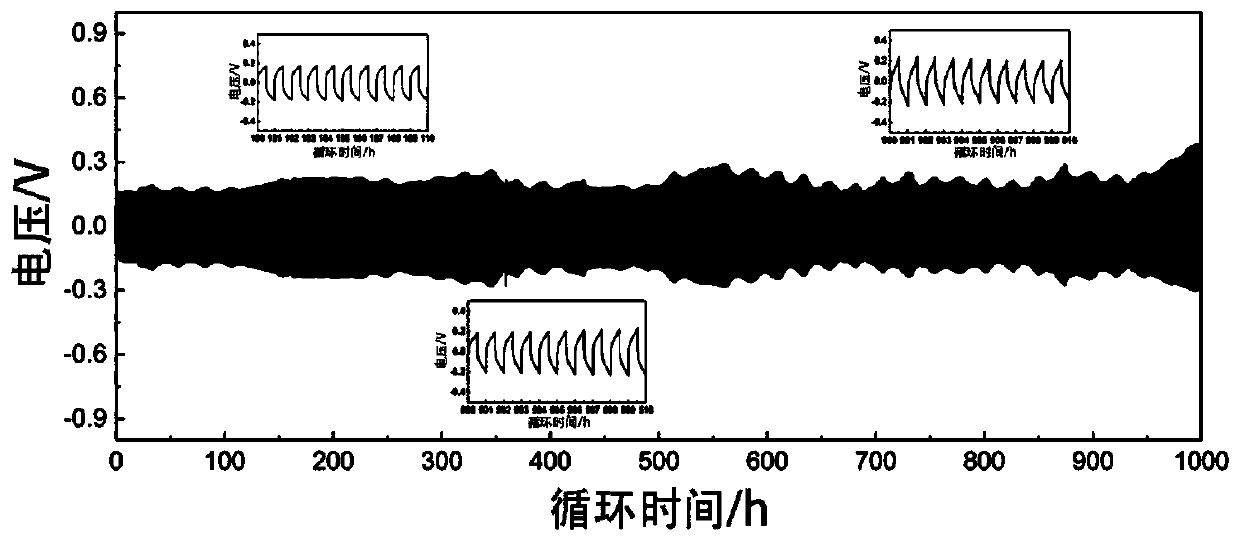
[0068] as attached figure 1 As shown, the conductivity of the obtained solid electrolyte is 1.8×10 -4 S / cm; as attached figure 2 As shown, the electrochemical window of the solid electrolyte obtained in the present invention can reach more than 6V. as attached image 3 It was shown that the resulting electrolyte was assembled into a Li-Li symmetric battery, and at 0.5mA cm -2 Under a high current density, it can be cycled stably for mo...
Embodiment 2
[0076] Preparation of the precursor: mix the polymer monomer polyethylene glycol dimethacrylate and the crosslinking agent tetraethylene glycol diacrylate in a ratio of 4:1, then add 1mol / L LiTFSI and 2wt% heat Initiator azobisisobutyronitrile until completely dissolved. The subsequent addition of 10 wt% ceramic electrolyte Li 6.25 Ga 0.25 La 3 Zr 2 o 12 Filler, stir until evenly dispersed, and then a completely mixed and uniform liquid precursor can be obtained. The precursor can form a solid electrolyte under thermal initiation.
[0077] as attached figure 1 As shown, the conductivity of the obtained solid electrolyte is 1.2×10 -4 S / cm.
[0078] In situ curing assembled battery: take 10 μL of the obtained precursor, carefully drop on the LiNi 0.6 co 0.2 mn 0.2 o 2 Between the composite positive electrode and the lithium metal negative electrode, it was then carefully moved to a heating device at 100 °C to initiate polymerization. After 90s, the integrated lamina...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com