Patents
Literature
160 results about "Isotope separation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
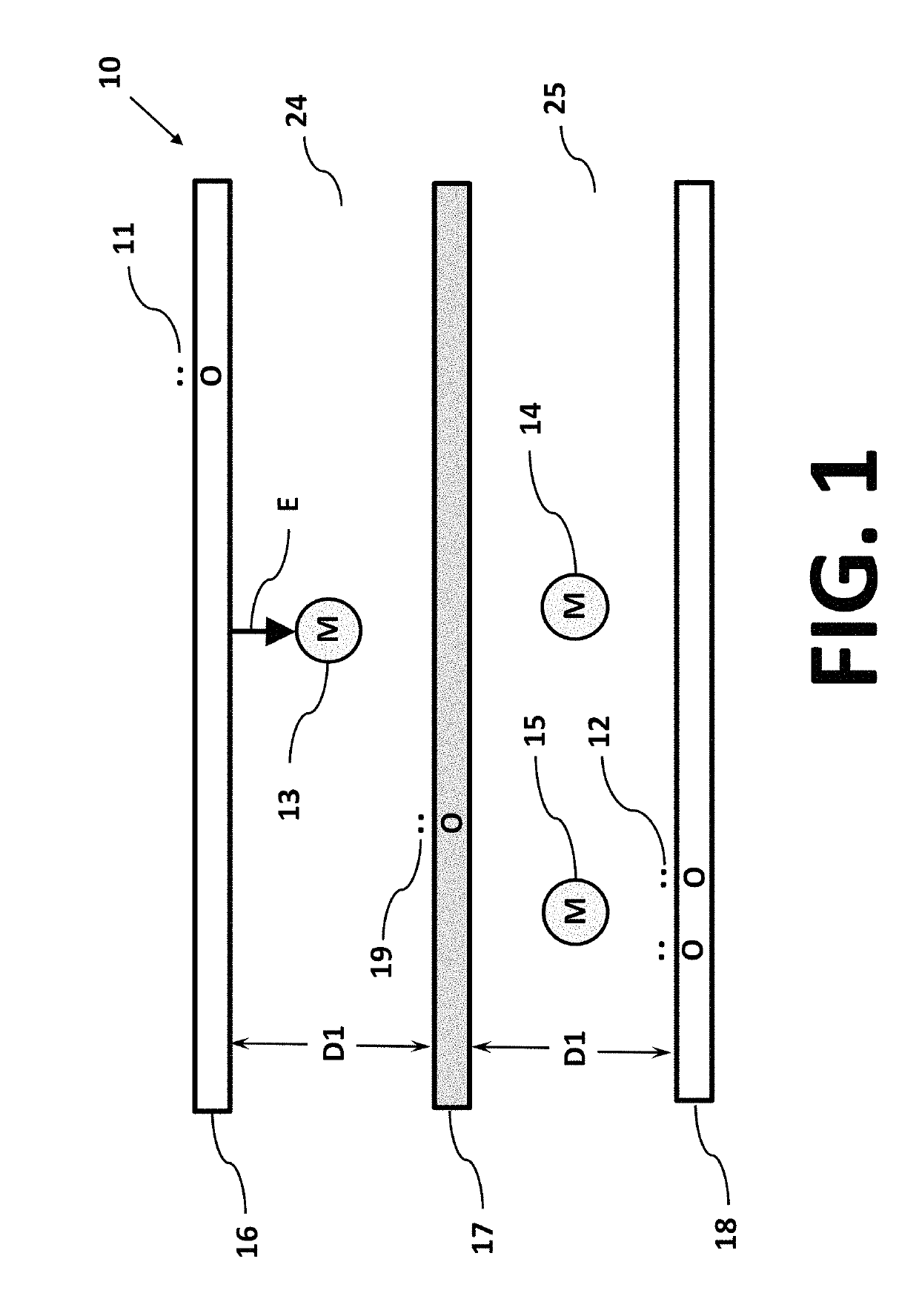
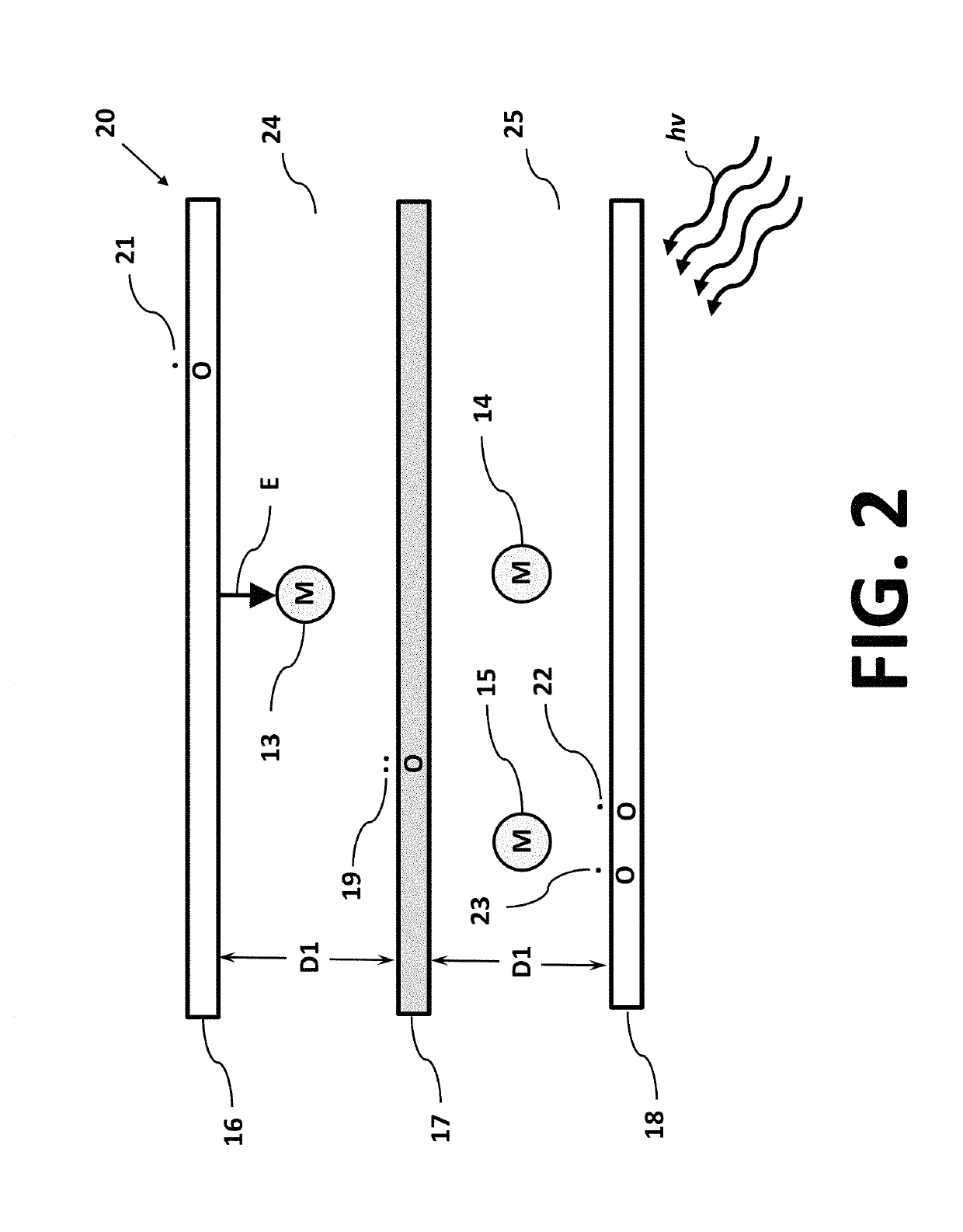
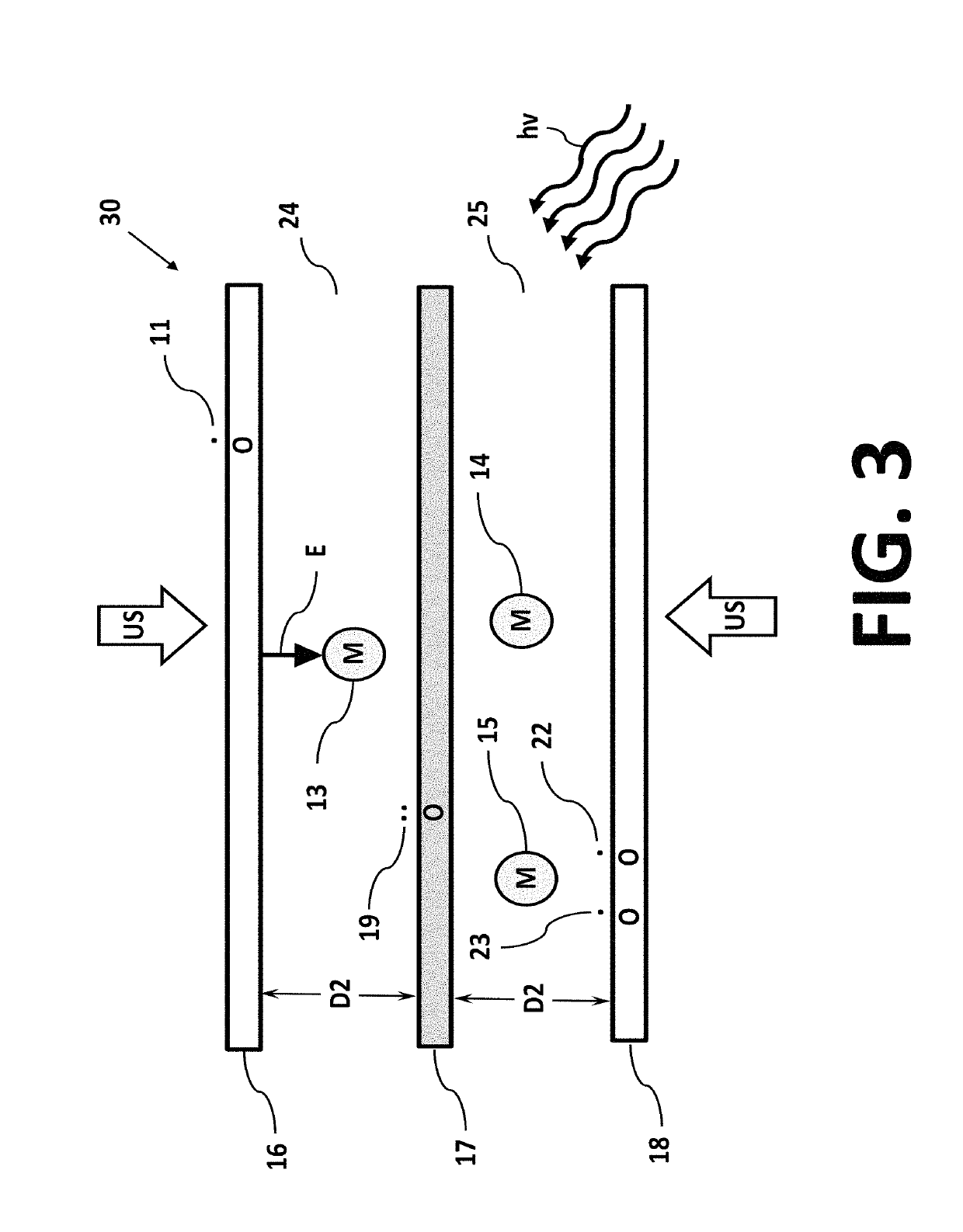
Isotope separation is the process of concentrating specific isotopes of a chemical element by removing other isotopes. The use of the nuclides produced is various. The largest variety is used in research (e.g. in chemistry where atoms of "marker" nuclide are used to figure out reaction mechanisms). By tonnage, separating natural uranium into enriched uranium and depleted uranium is the largest application. In the following text, mainly the uranium enrichment is considered. This process is a crucial one in the manufacture of uranium fuel for nuclear power stations, and is also required for the creation of uranium based nuclear weapons. Plutonium-based weapons use plutonium produced in a nuclear reactor, which must be operated in such a way as to produce plutonium already of suitable isotopic mix or grade. While different chemical elements can be purified through chemical processes, isotopes of the same element have nearly identical chemical properties, which makes this type of separation impractical, except for separation of deuterium.
Non-woven base composite membrane for lithium isotope separation, and preparation method thereof, as well as lithium isotope separation method by using membrane chromatography
InactiveCN103386299AAchieve serializationSerialization is easy to implementOther chemical processesSolid sorbent liquid separationChromatographic separationPolymer solution
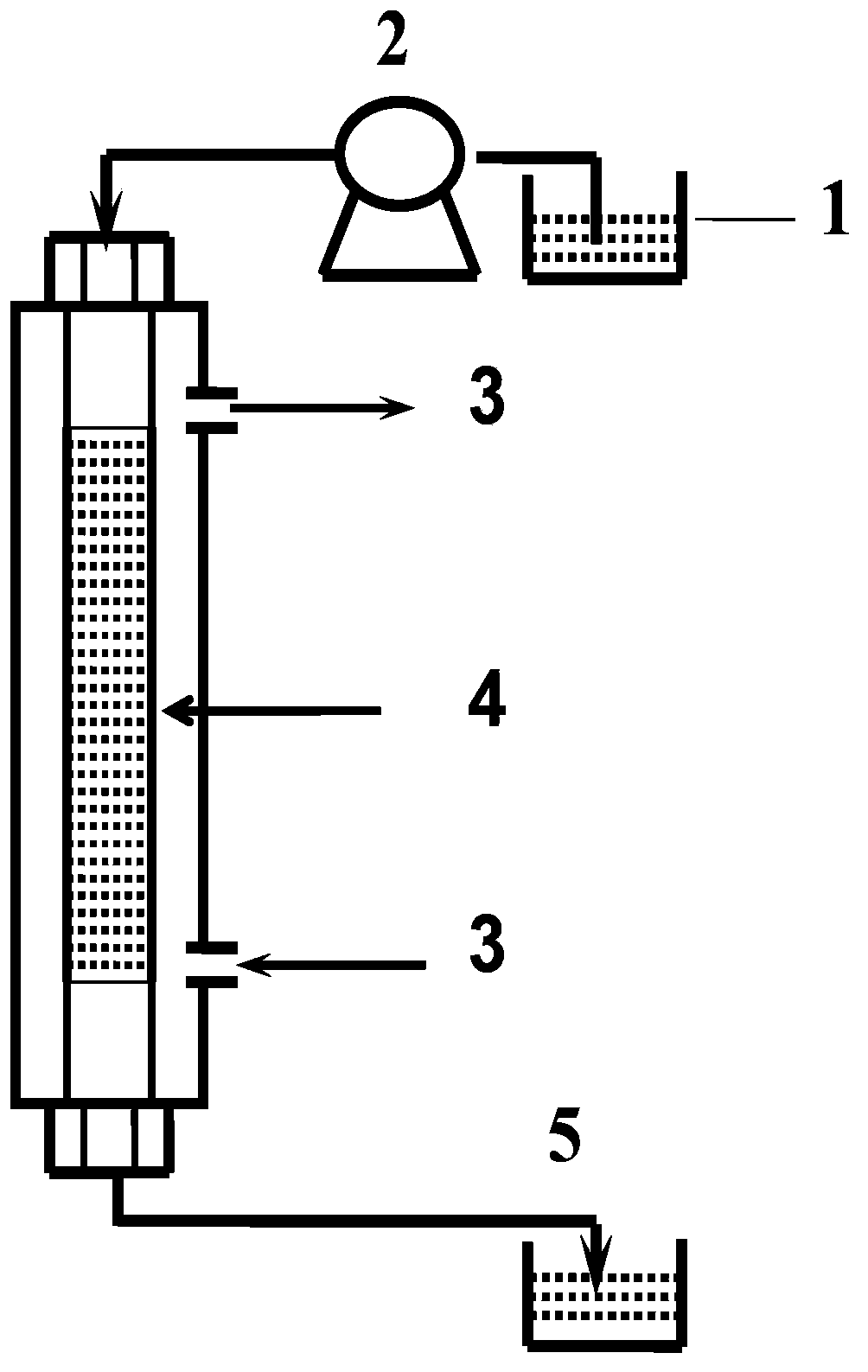
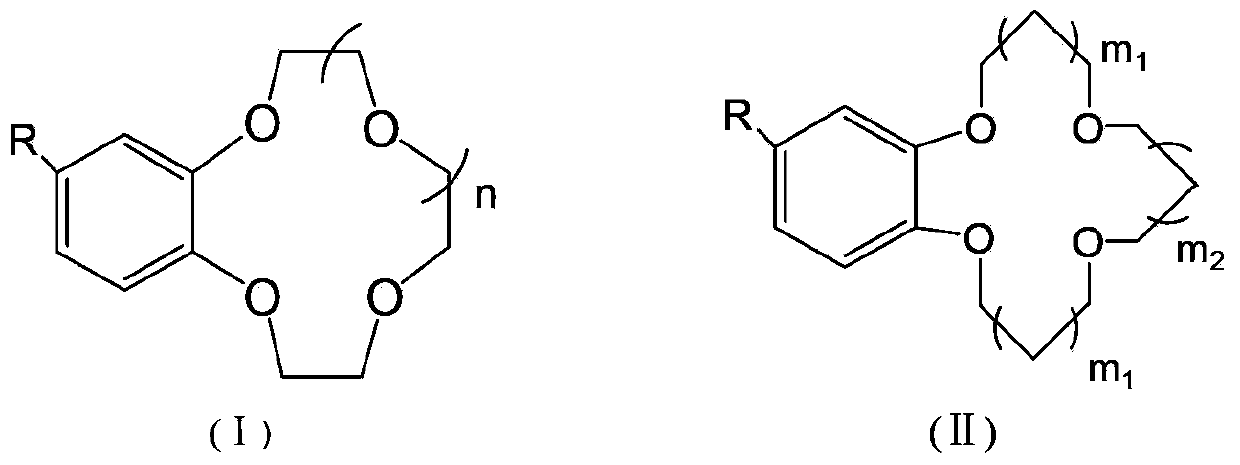
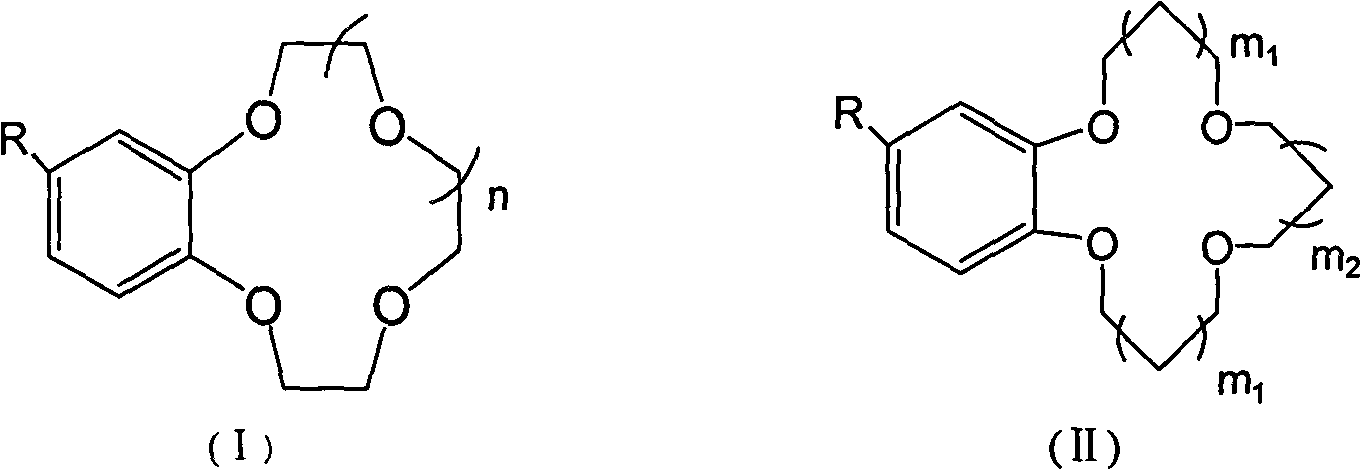
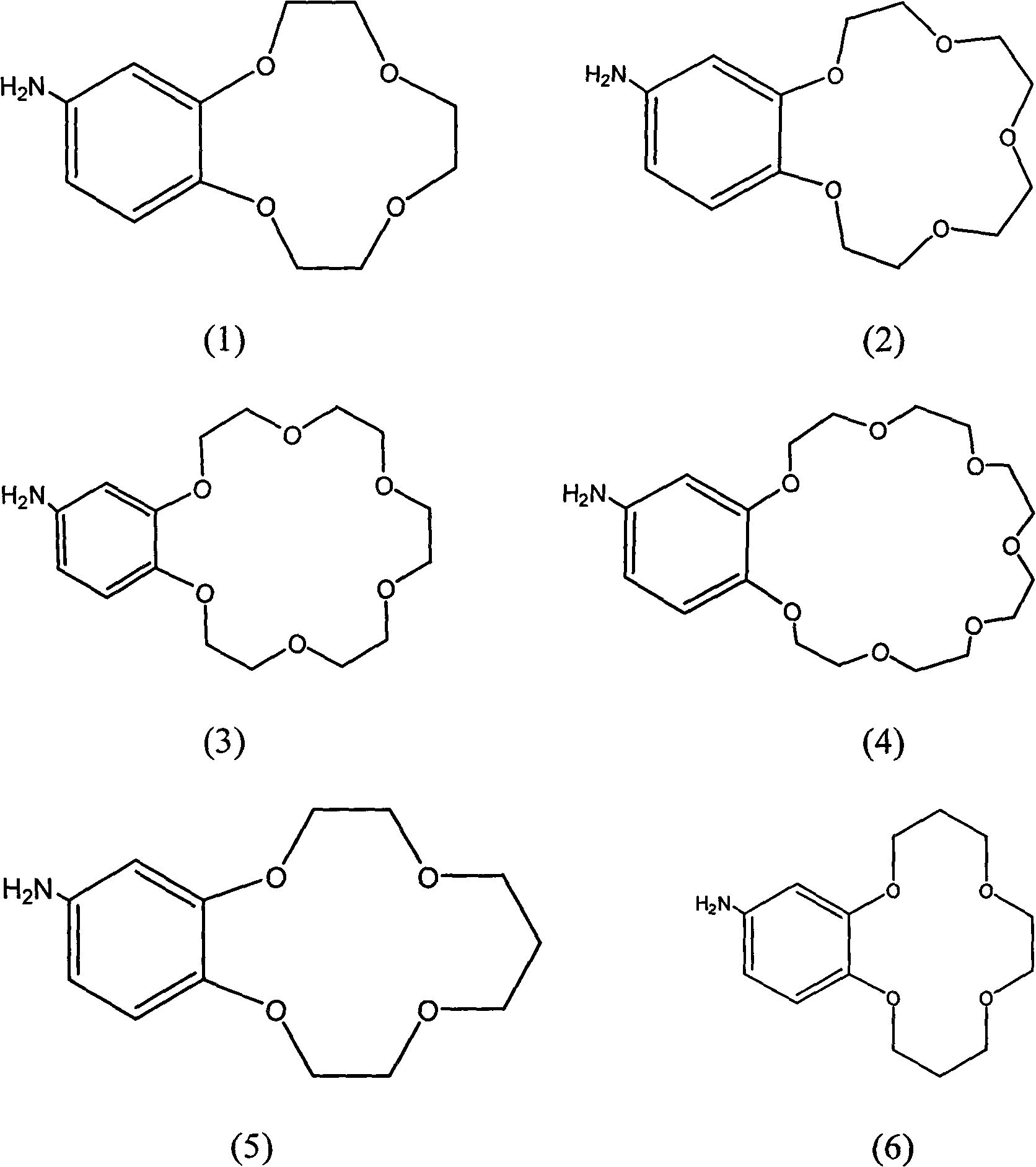
The invention provides a non-woven base composite membrane for lithium isotope separation and a preparation method thereof, as well as a lithium isotope separation method by using a membrane chromatography. The non-woven base composite membrane takes a non-woven fabric as a porous support body or a micropore membrane, a coating is prepared on the non-woven fabric by taking a crown ether graft polymer or a Kryptofix graft polymer with a lithium isotope separation effect as a film forming matter, or a coating can be prepared on the non-woven fabric by blending crown ether or Kryptofix and derivates of the crown ether or the Kryptofix into a film-formation polymer solution, and the coating and a base membrane are combined to form a composite separated membrane; the composite separated membrane is taken as a membrane chromatography medium stationary phase, so as to separate lithium isotopes by using the membrane chromatography. The non-woven base composite membrane or the lithium isotope separation method by using the membrane chromatography can effectively improve the contact and coupling efficiency of crown ether molecules and Lithium Ions in the separation process for solid-liquid extraction lithium isotopes, and realize the greenization, serialization and high efficient of the process of lithium isotope separation.
Owner:TIANJIN POLYTECHNIC UNIV
Benzo crown ether graft polymer material with lithium isotope separation effect and preparation method thereof
InactiveCN102911372AHigh crown ether immobilization capacityExcellent separation factorMethyl groupPolymer chemistry
Owner:TIANJIN POLYTECHNIC UNIV
Hypothermia distillation device and method for separating stable isotopes
InactiveCN1962037ALittle operational flexibilityLarger drag dropIsotope separationDistillationVolumetric Mass Density
The invention relates to a low-temperature finestiller for separating stable isotope elements, wherein it comprises at least one low-temperature finestill tower, which is packed with multilayer thermal-insulated layer at high vacuum; the tower is filled with multilayer stuff; the density of multilayer thermal-insulated material is 10-150layer / cm; the vacuum of thermal-insulated layer is lower than 10-1Pa; the stuff is wave stuff as plate wave stuff and mesh wave stuff as best. The inventive separating method comprises processing low-temperature gas-liquid conversation in the low-temperature finestill tower, to separate the isotope elements. The invention can be used in CO, CH4, NO, BF3, O2, H2, He, or Ne to separate 3CO, 3CH4, C18O, 18OO, 15NO, N18O, 10BF3, 2H, 3H, 3He, 22Ne, 12CO, 12CH4, N16O, 14NO, 11BF3, and 20Ne.
Owner:SHANGHAI RES INST OF CHEM IND
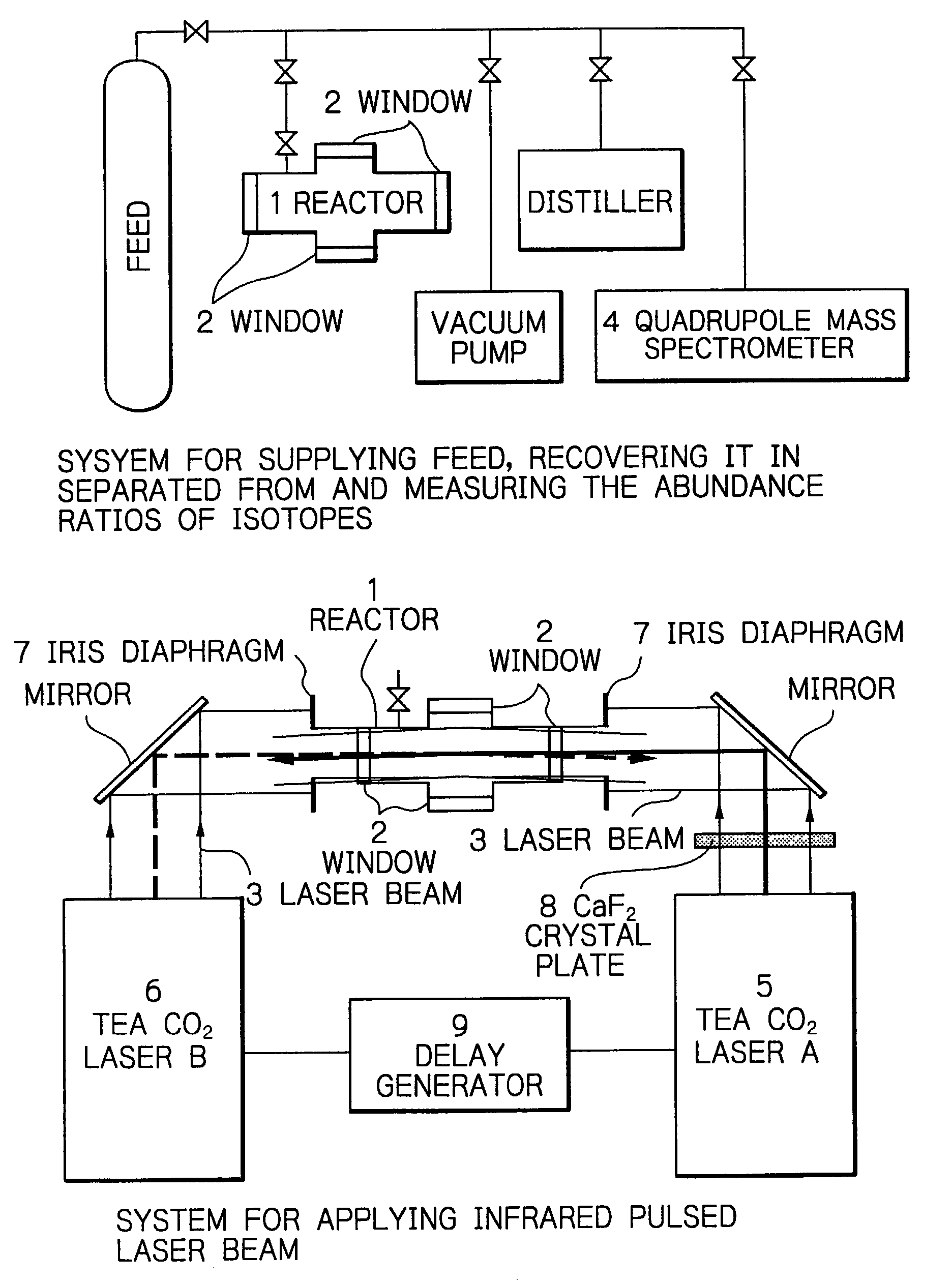
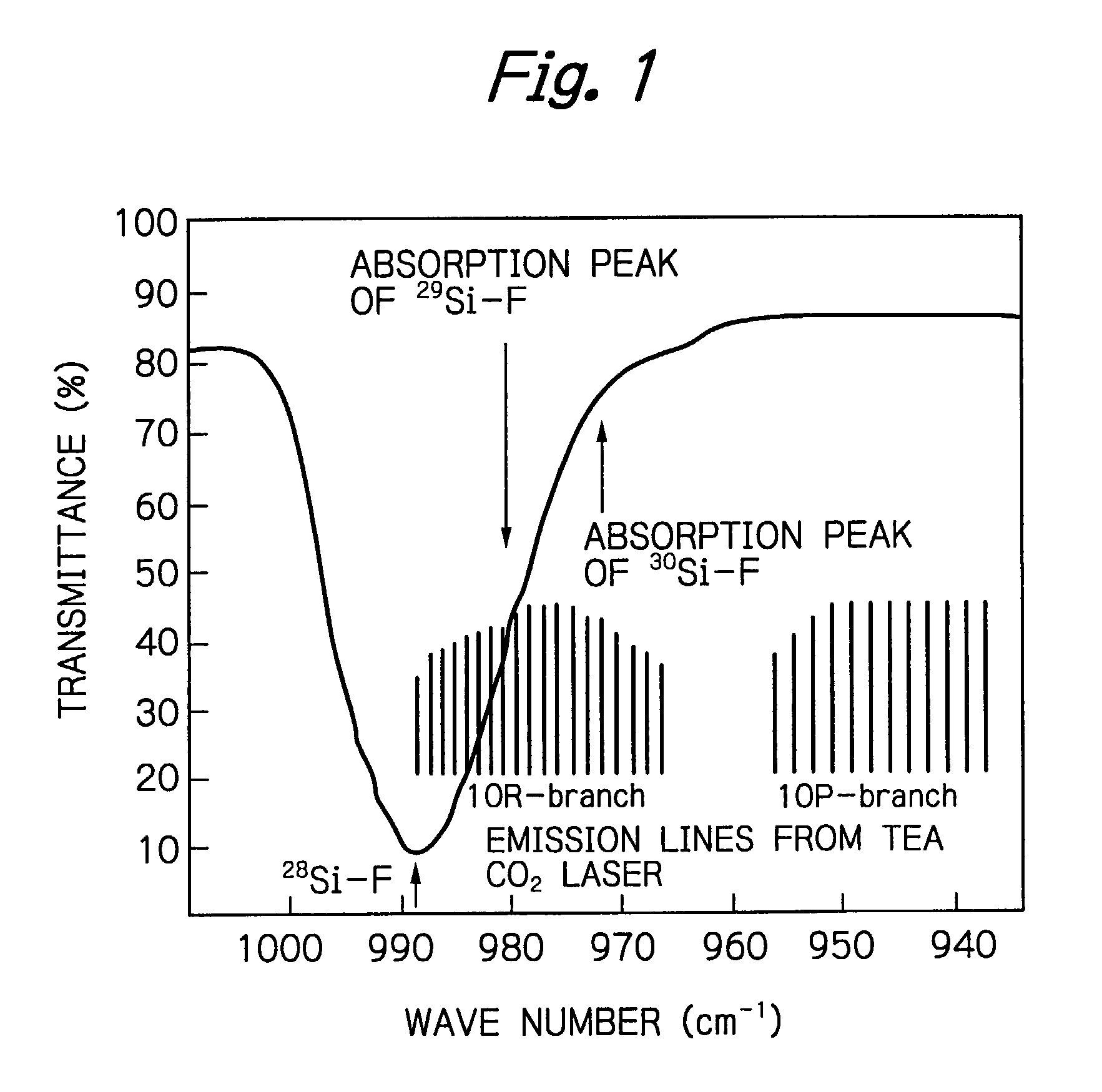
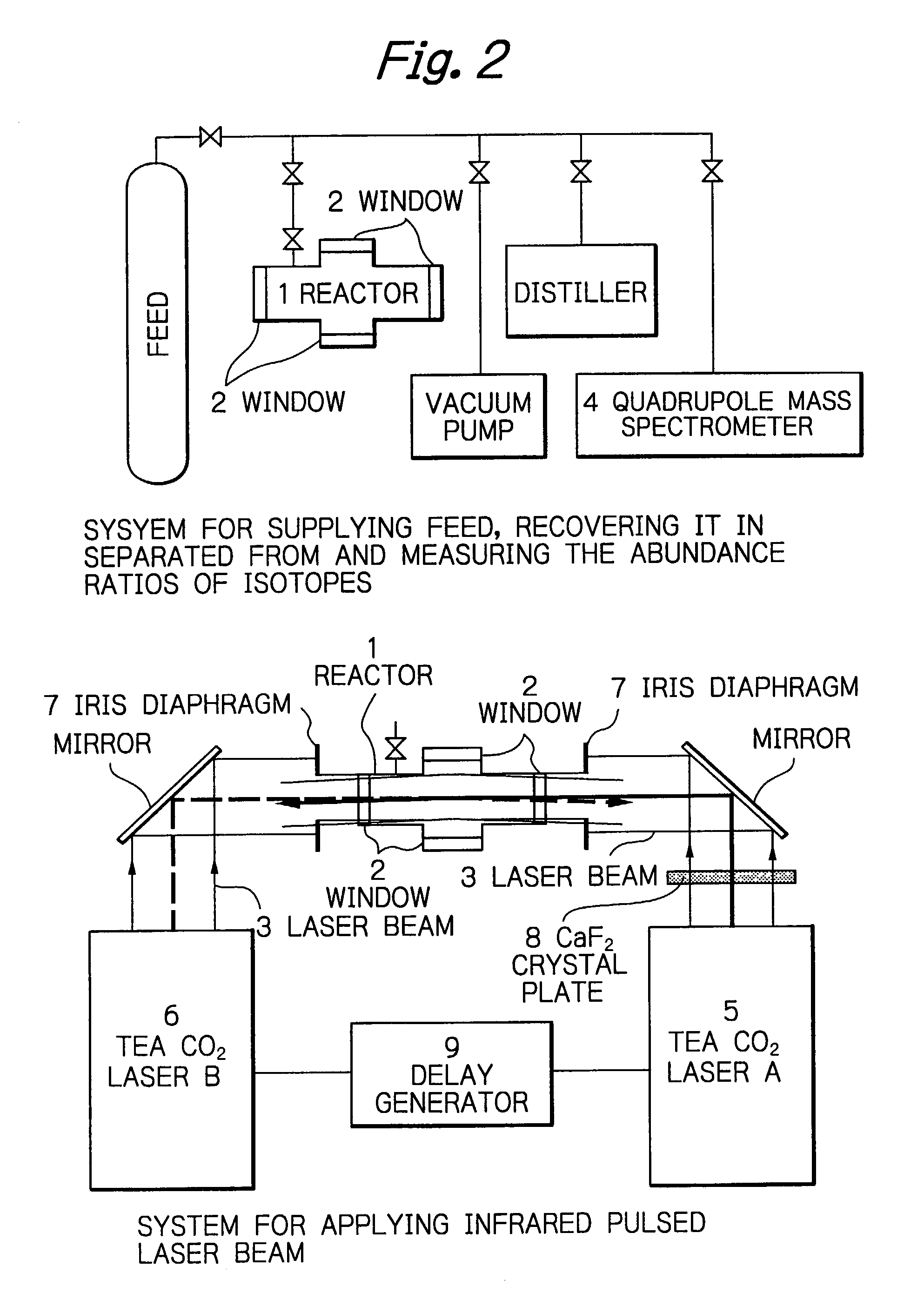
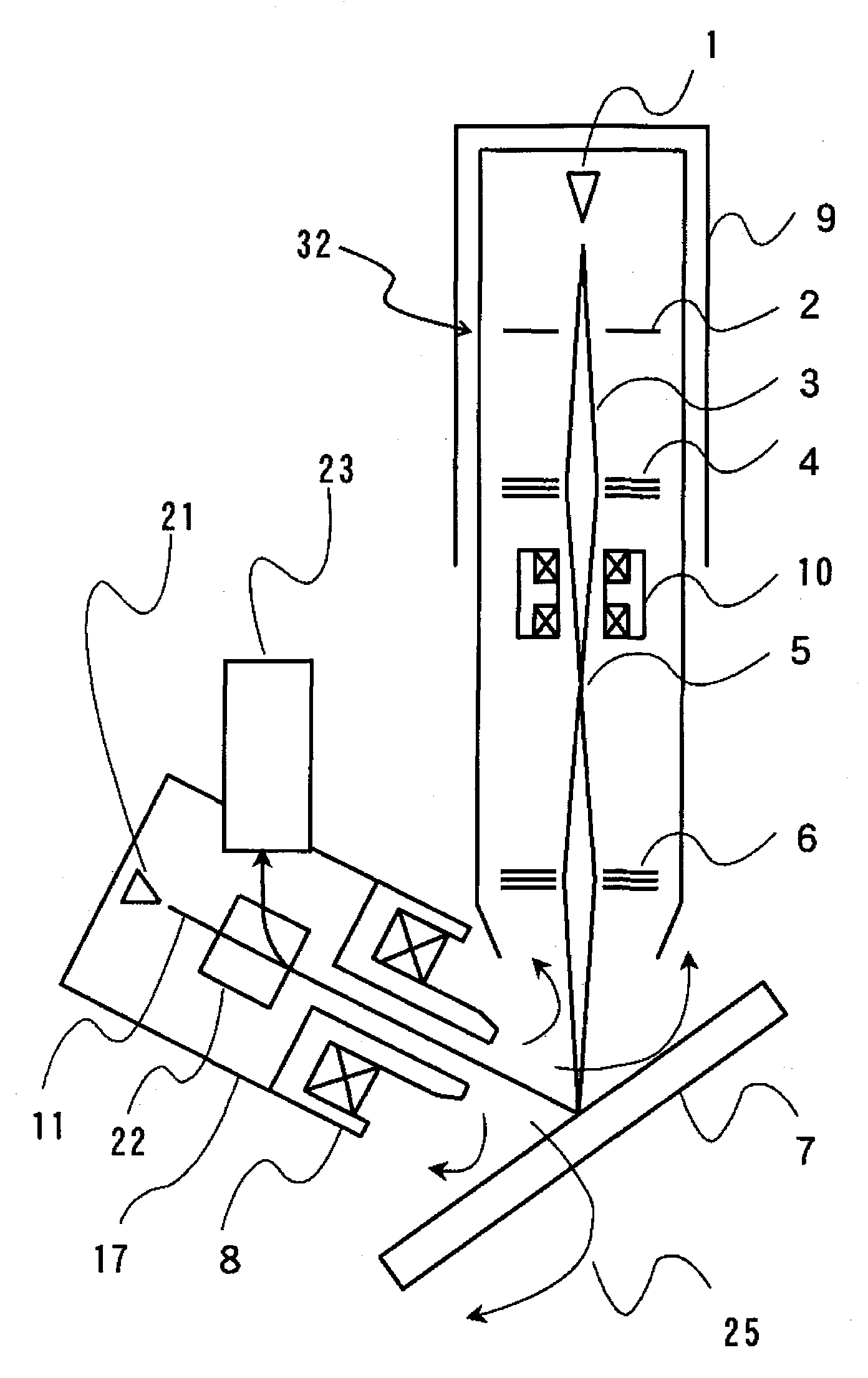
Method for efficient laser isotope separation and enrichment of silicon
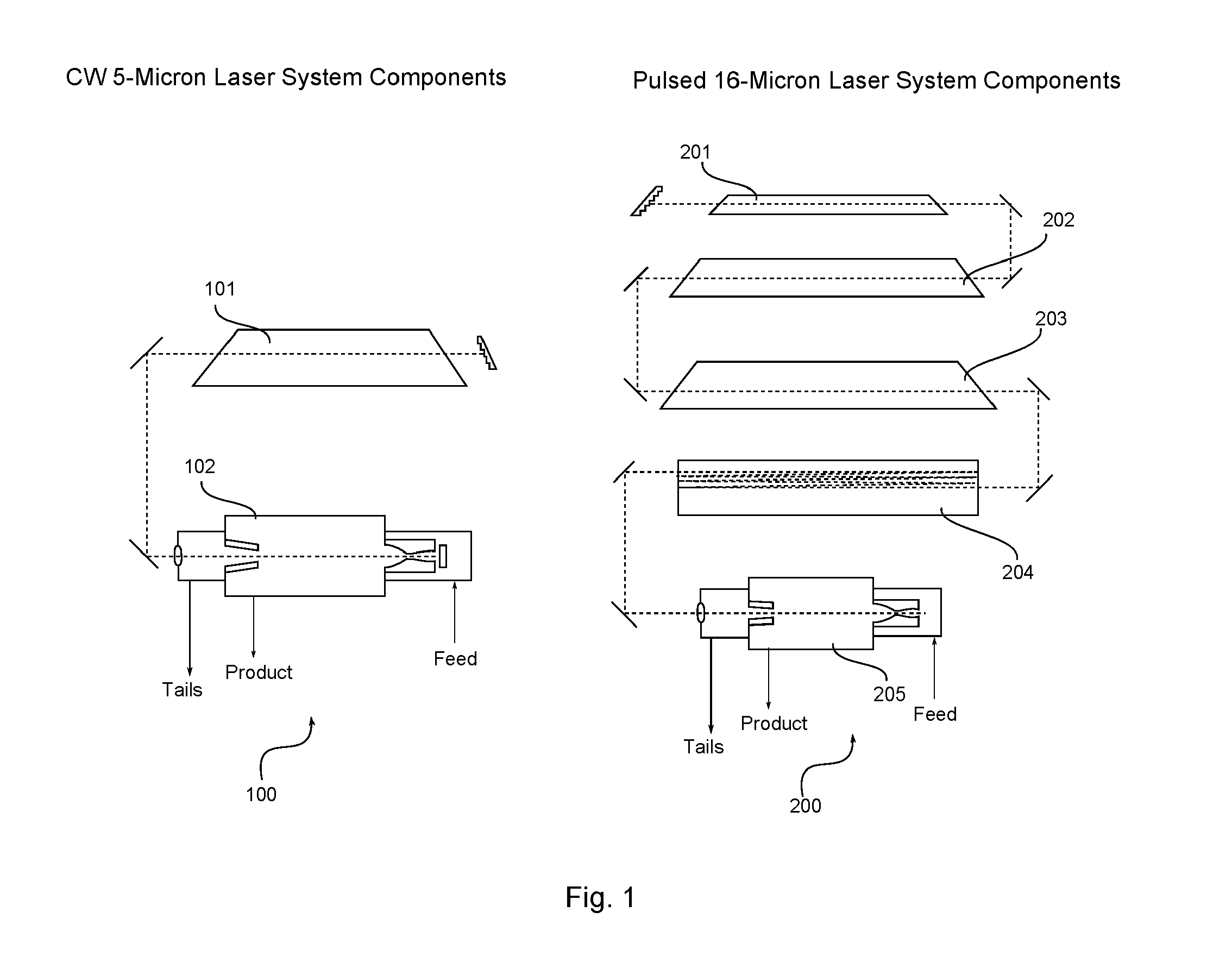
InactiveUS20030034243A1Improve efficiencyStrong absorption capacityVapor condensationIsotope separationLaser assistedPulsed laser beam
A method for laser-assisted separation and enrichment of silicon isotopes such as 28Si, 29Si and 30Si on the basis of infrared multiple-photon dissociation of silicon halides represented by Si2F6, wherein the silicon halides are irradiated synchronously with multiple infrared pulsed laser beams at different wavelengths.
Owner:JAPAN ATOM ENERGY RES INST
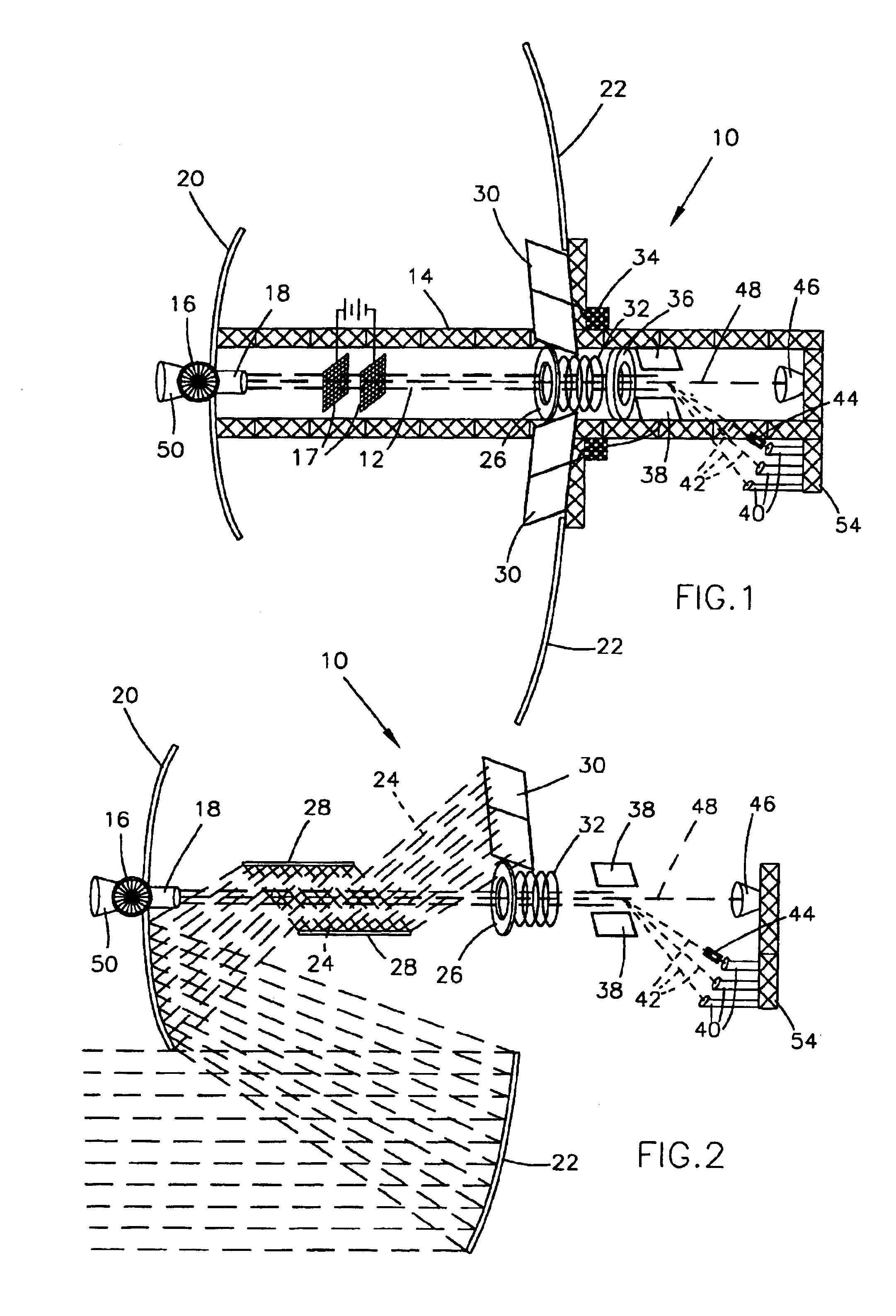
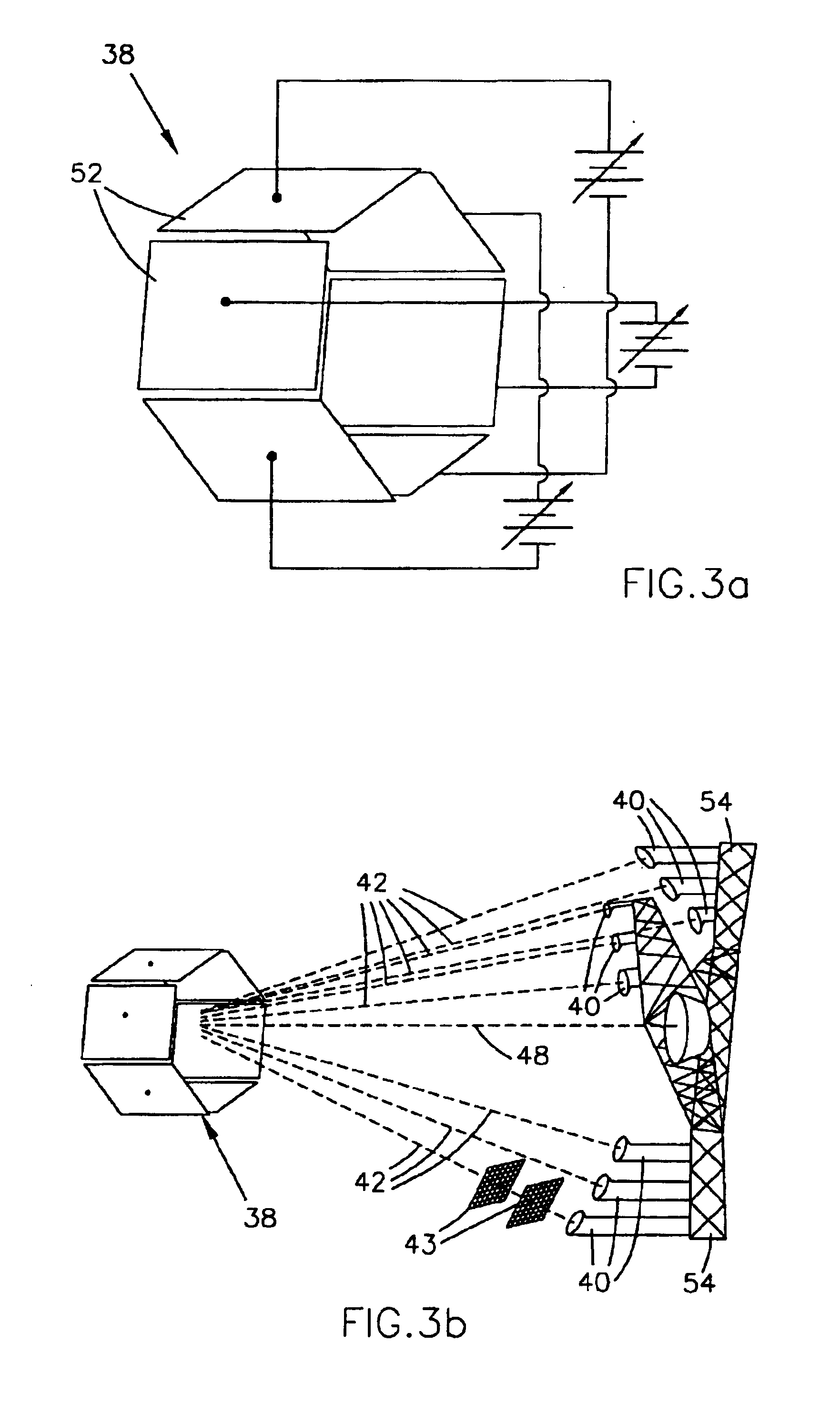
Focused ION Beam Apparatus
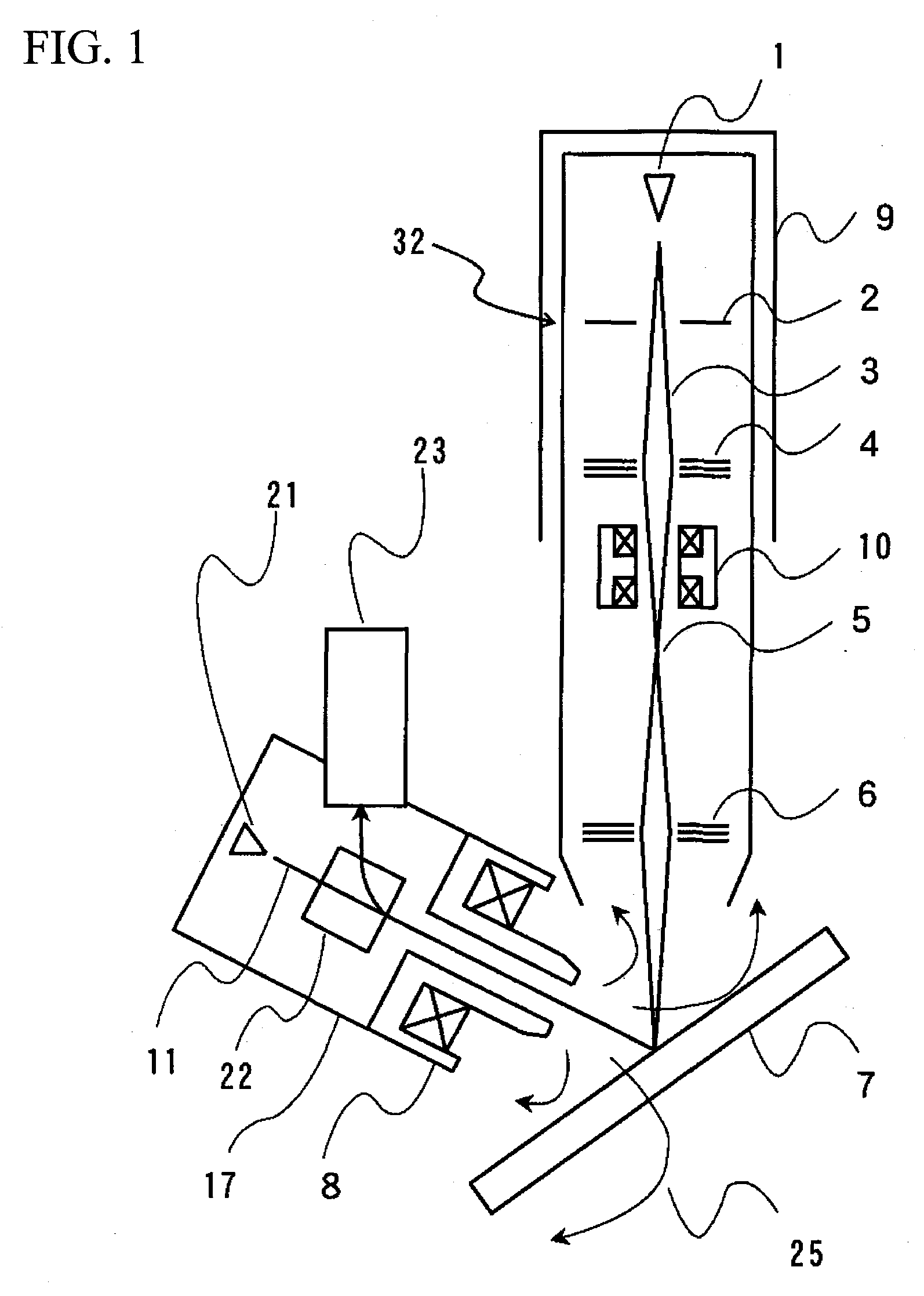
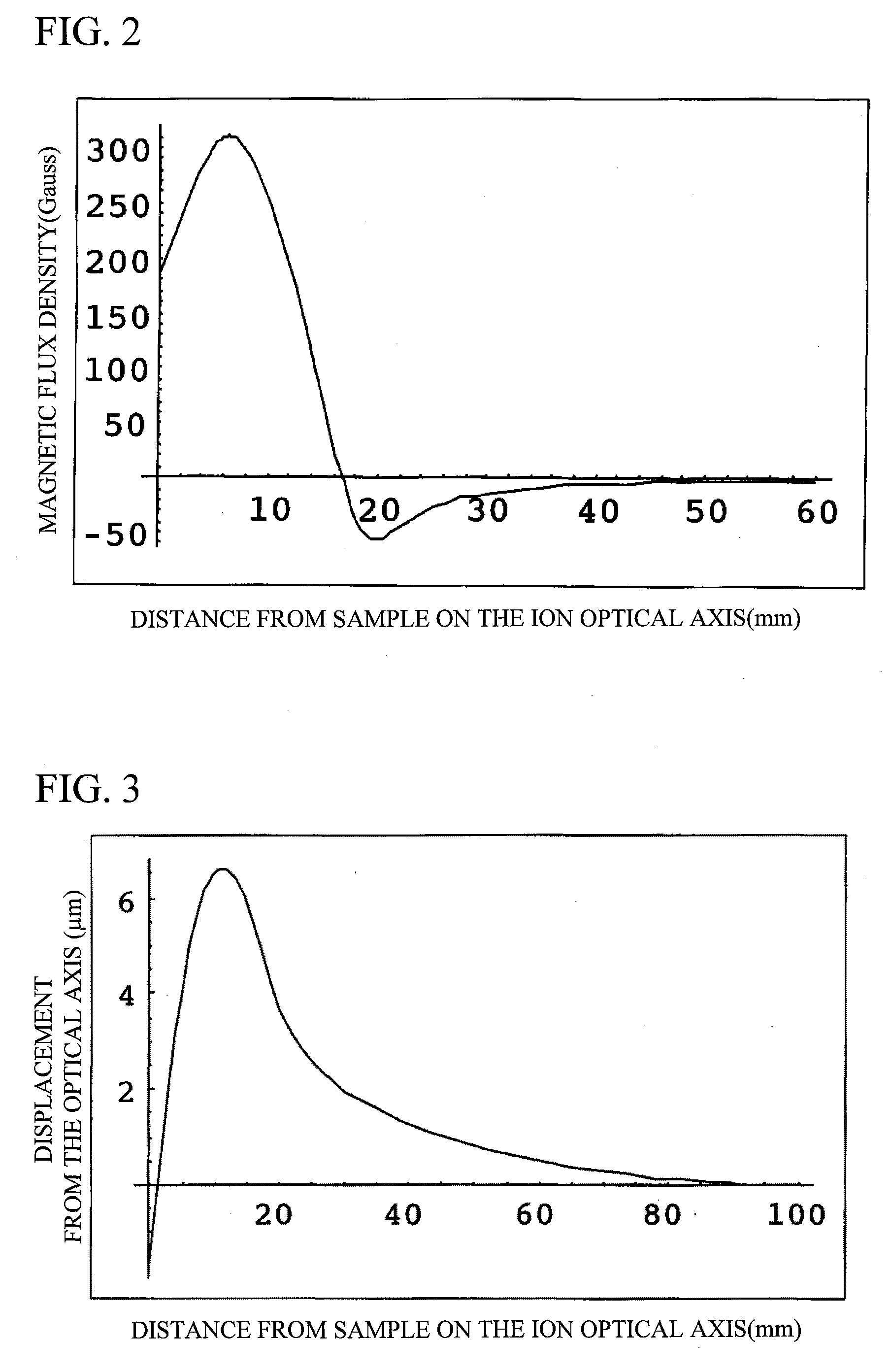
InactiveUS20080023641A1Radiation/particle handlingMaterial analysis by optical meansOptical axisIon beam
A focused ion beam apparatus enables an ion beam to be focused highly accurately on a sample at the beam spot position of the case of the absence of magnetic field without causing isotope separation of the ion beam on the sample, even when there is a magnetic field on the ion beam optical axis or the magnetic field fluctuates. The focused ion beam apparatus comprises a corrective magnetic field generating unit 10 disposed on the optical axis of the ion beam 3 for correcting the deflection of the ion beam 3 due to an external magnetic field. The corrective magnetic field generating unit 10 includes pole-piece pairs 26A and 26B, each of which having two pole pieces 26a and 26b or 26c and 26d that are adjacent to each other with a gap d. The pole-piece pairs 26A and 26B are disposed opposite to each other with a gap g (>d) across the optical axis of the ion beam 3. Each of the pole pieces 26a to 26d is wound with an internal coil 29, and the pole-piece pairs 26A and 26B are each wound with an external coil 30 in such a manner as to surround the internal coils 29.
Owner:HITACHI HIGH-TECH CORP
Method for separating lithium isotopes by coupling electric field and crown ether graft polymer porous membrane
The invention provides a method for separating lithium isotopes by coupling an electric field and a crown ether graft polymer porous membrane. According to the method, a lithium isotope separation effect of the crown ether functionalized polymer membrane and a lithium isotope separation effect under the action of the electric field are coupled so as to enhance the lithium isotope separation effectof a system; specifically, the crown ether functionalized porous membrane with the lithium isotope separation effect is fixed in the middle of a diffusion cell, the two sides of the diffusion cell are provided with a lithium salt solution and pure water respectively, the selective adsorption effect of the functionalized membrane on <7>Li<+> is utilized, and <6>Li<+> is enriched on the pure waterside; and meanwhile, the <6>Li<+> has a higher migration rate under the action of the electric field, so that the migration rate of <6>Li<+> isotope ions towards the pure water side is increased, andfinally, green and efficient lithium isotope ion separation is realized.
Owner:TIANJIN POLYTECHNIC UNIV
Method for stable oxygen isotope separation and its apparatus using membrane distillation
InactiveUS7638059B2High separation factorGood effectSolidificationLiquefactionRefluxChemical physics
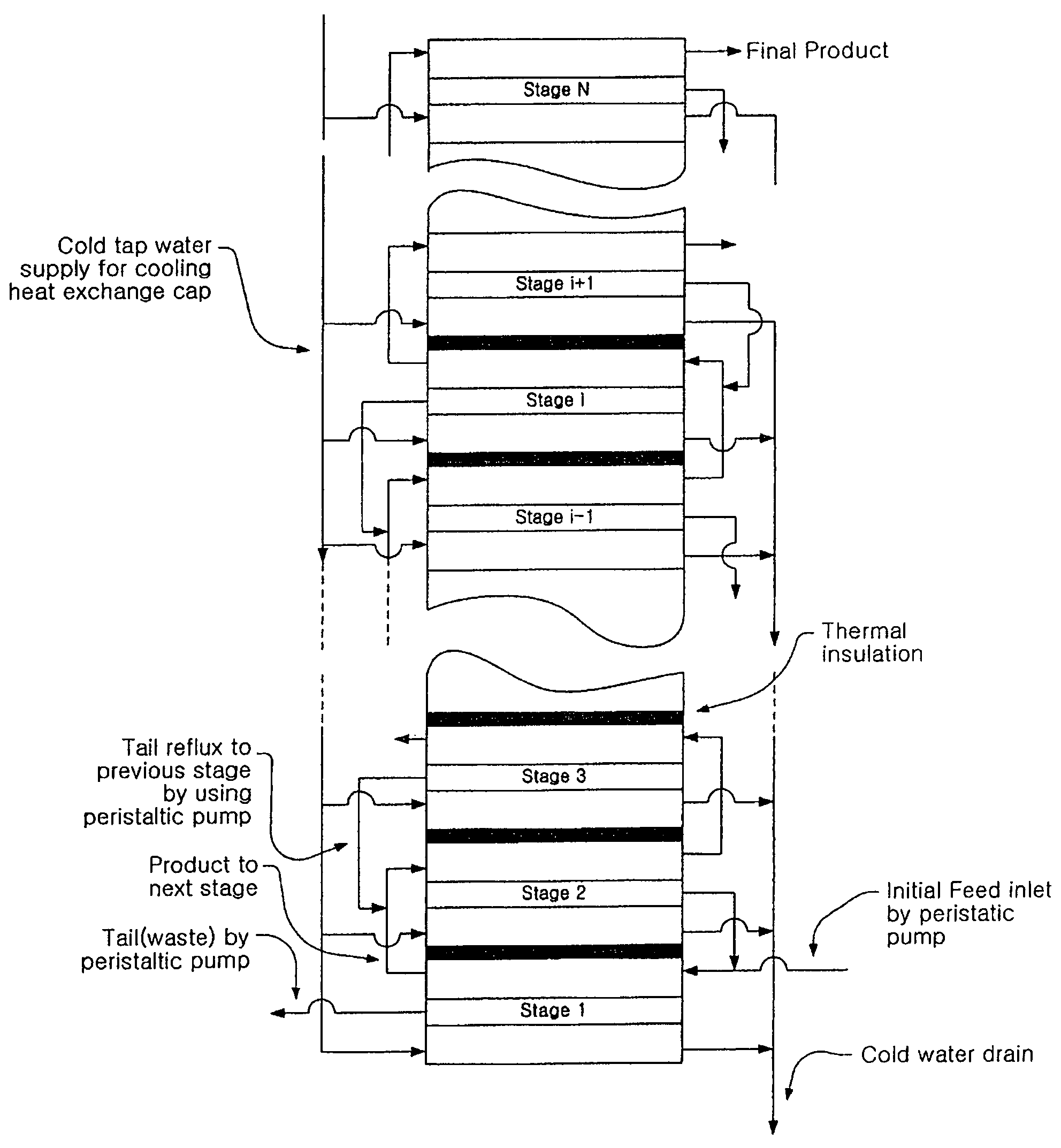
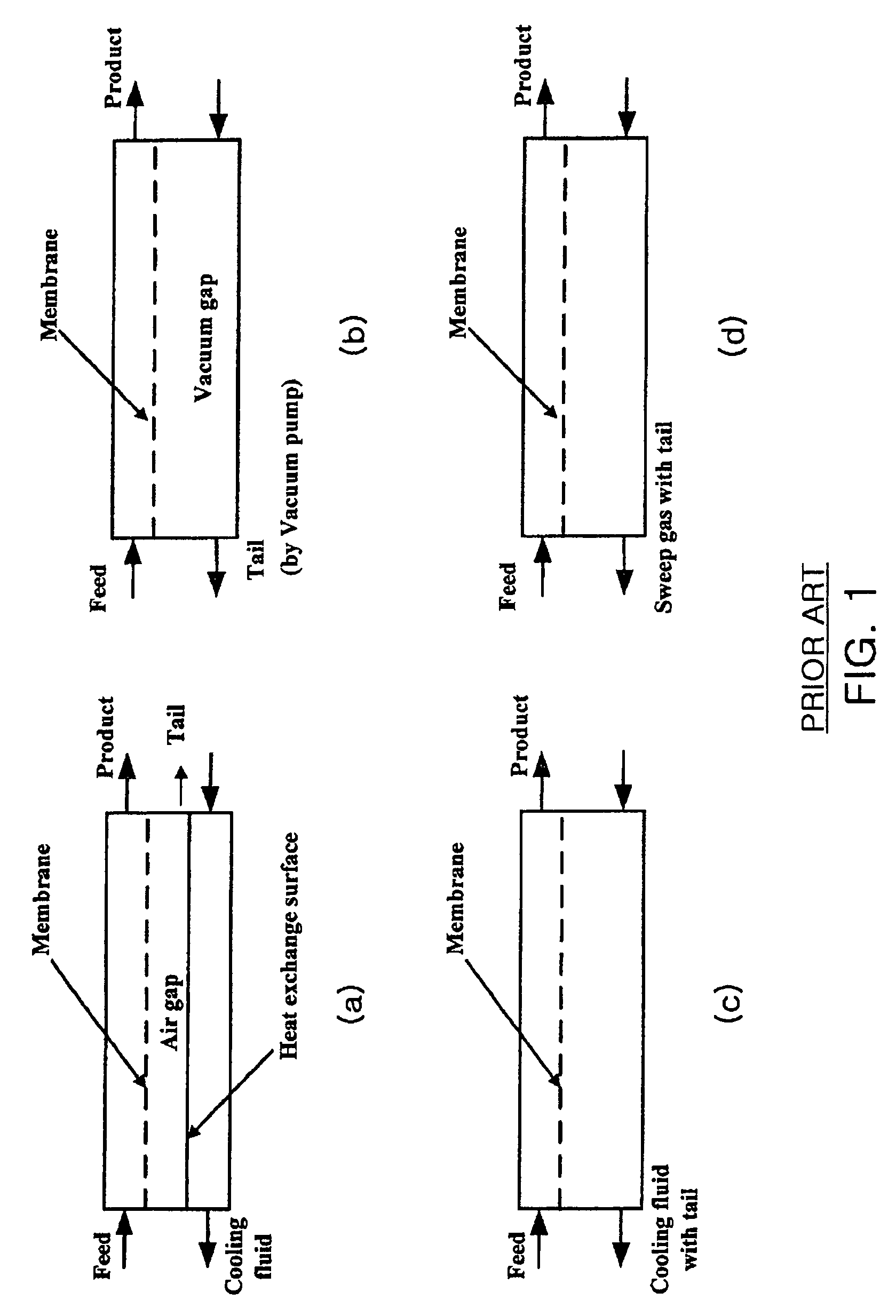
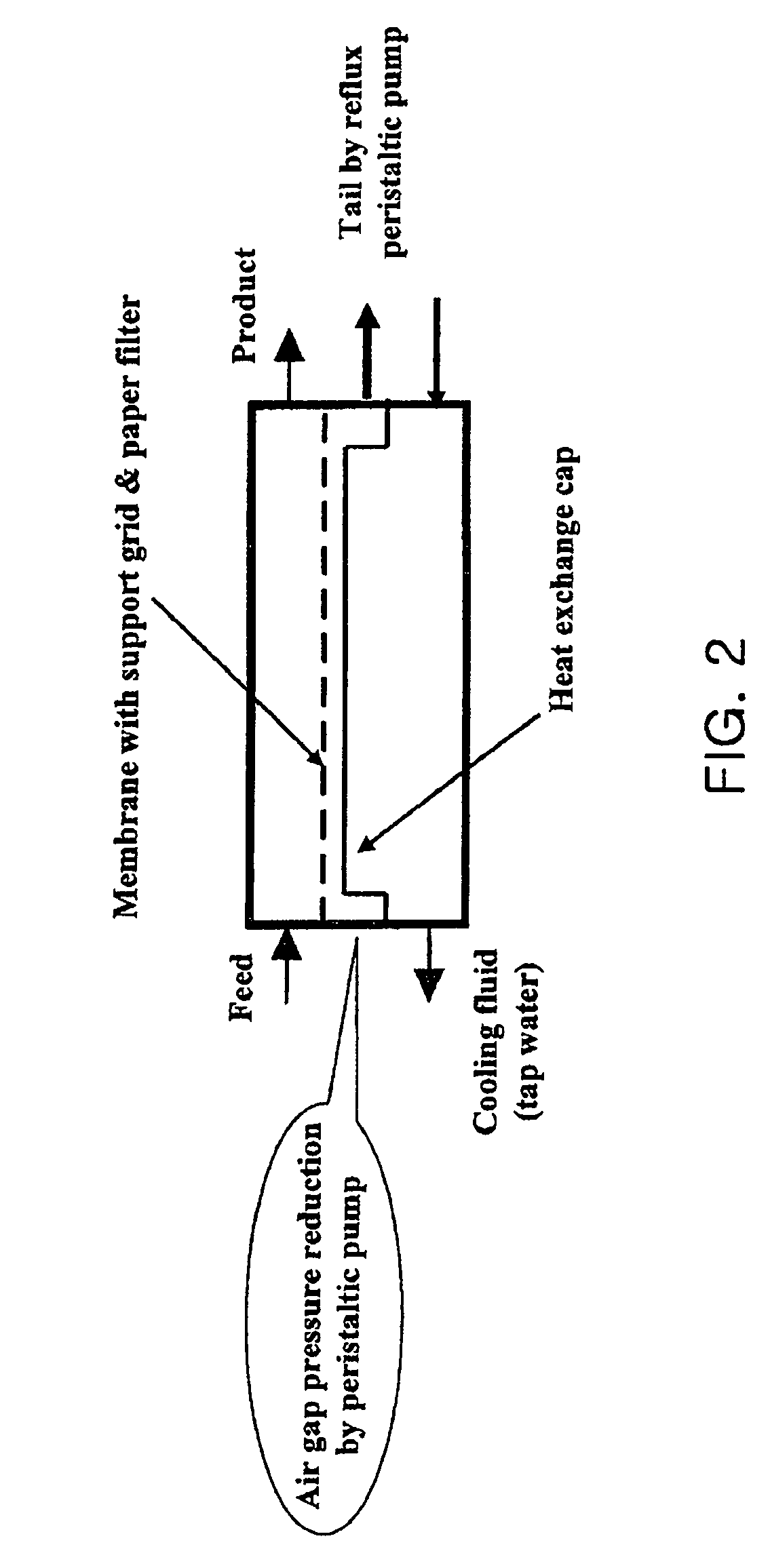
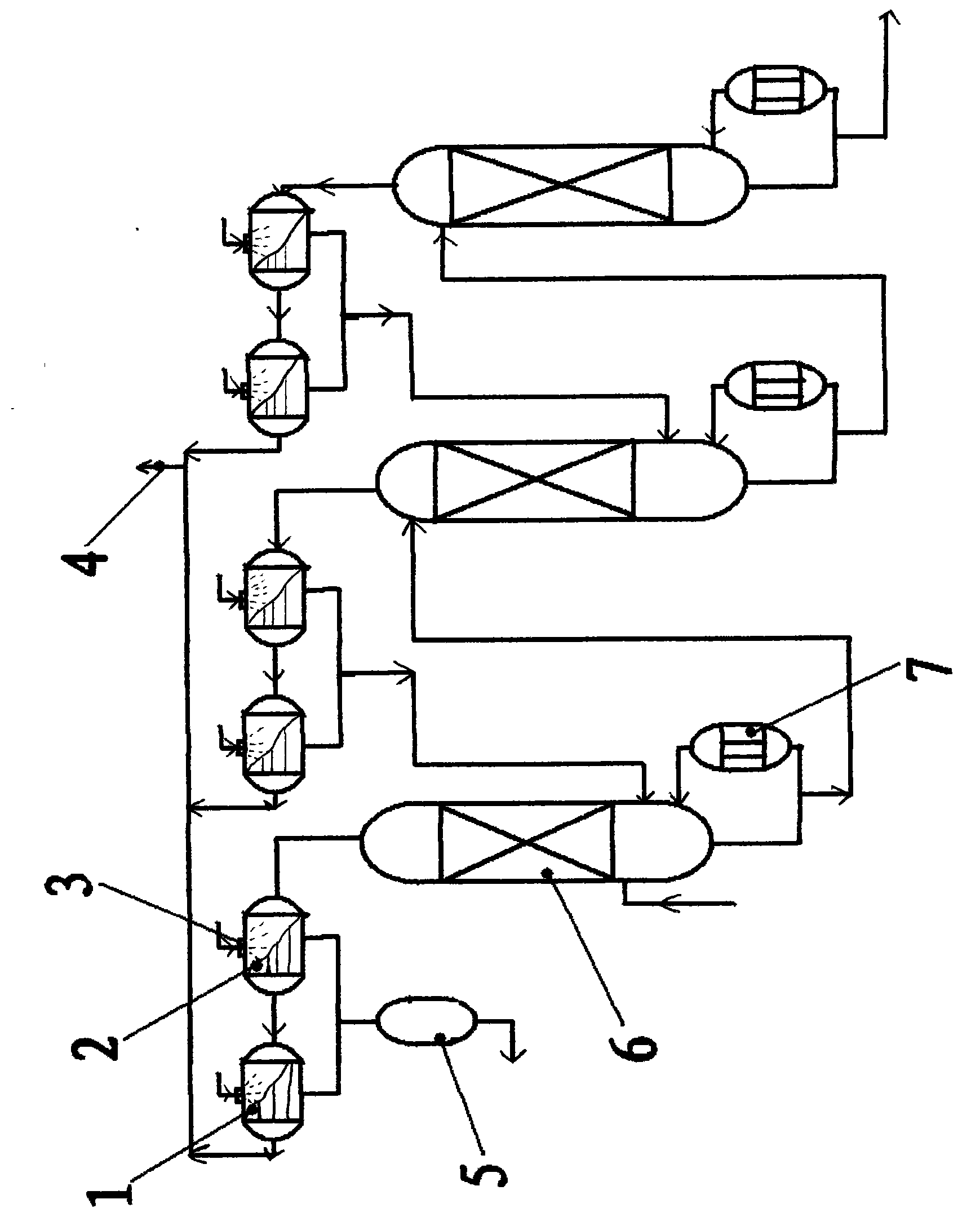
The present invention relates to an oxygen isotope separation system and a method therefor. More specifically, the invention relates to a newly invented pressure-driven AGMD (Air Gap Membrane Distillation) system applied to a multi-stage membrane distillation cells which can produce an oxygen isotope effectively and economically, and a method therefor. The invention provides an oxygen isotope separation system including a number of Air Gap Membrane Distillation (AGMD) permeation cells connected in series to separate a feed into a product and a tail, wherein each of the AGMD cell is connected at a tail outlet with a reflux pump and at a product outlet, whereby the product from (i−1)th cell and the tail from (i+1)th cell are pumped as the feed into ith cell.
Owner:KOREA ATOMIC ENERGY RES INST
Method for preparing ultralight water by isotope distillation
The invention relates to a method for preparing ultralight water by isotope distillation, belonging to the technical field of isotope distillation and comprising the following steps: preparing purified water with city tap water as the raw material water which is purified to obtain purified water; and preparing ultralight water, i.e. introducing an isotope separator to carry out isotope mass transfer exchange so as to obtain ultralight water with deuterium content of 1-100 ppm. The method is beneficial to improving the isotope separation effect, has simple step and convenient operation, and can use the isotope separator according to the content of deuterium required, thereby showing ideal separation efficiency and shortening theseparation time.
Owner:江苏奥特泉超轻水饮料有限公司
Method for measuring isotope ratio of uranium in single particle
ActiveCN104236978AThe positioning method is simple and fastImprove efficiencyPreparing sample for investigationMaterial analysis by electric/magnetic meansLaser ablation inductively coupled plasma mass spectrometryAdhesive
The invention belongs to the technical field of monitoring of nuclear technology, and discloses a method for measuring the isotope ratio of uranium in a single particle. The method comprises the following steps: firstly seeking a uranium-containing particle by using a scanning electron microscope, using a micro-operation system to transfer the uranium-containing particle to a conductive adhesive with a marked metal mesh on the surface, positioning and fixing the uranium-containing particle, and then analyzing the isotope ratio by using a laser ablation-inductively coupled plasma mass spectrometry. The method has the advantages of simple and quick pre-treatment method, simplified analysis flow, and high analysis precision which can reach to 1%.
Owner:CHINA INSTITUTE OF ATOMIC ENERGY
Site analysis system for the calculation of the isotope ratio of carbon in several gas species by means of a single analyser
InactiveUS20130064715A1Shorten the timeComponent separationMaterial analysis by electric/magnetic meansChemical elementMobile laboratory
The present invention relates to a system of analysis, which can be used in a mobile laboratory in a drilling site situation or in a similar situation, suitable for measuring (preferably in relation to at least two partially gaseous species, which derive preferably from a mixture extracted from a drilling mud, for example, methane, ethane, propane and / or any other heavier hydrocarbons) the quantities of the different isotopes of at least a same chemical element (preferably the quantities of 13C, carbon isotope with 6 protons and 7 neutrons, and of 12C, carbon isotope with 6 protons and 6 neutrons, respectively) by means of a laser isotopes analyser regulated for a single, at least partially gaseous species which contains said chemical element.
Owner:CALLERI ANTONIO
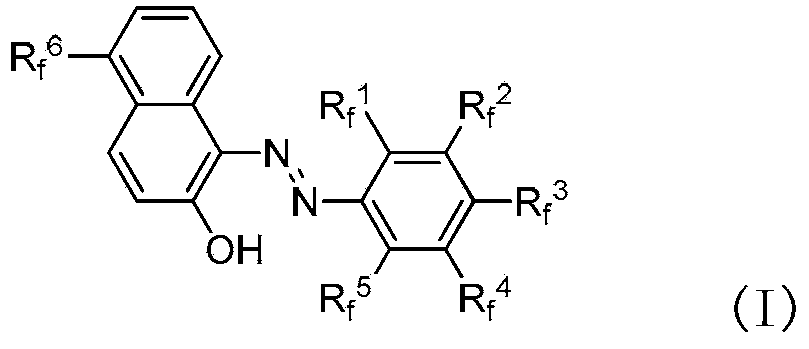
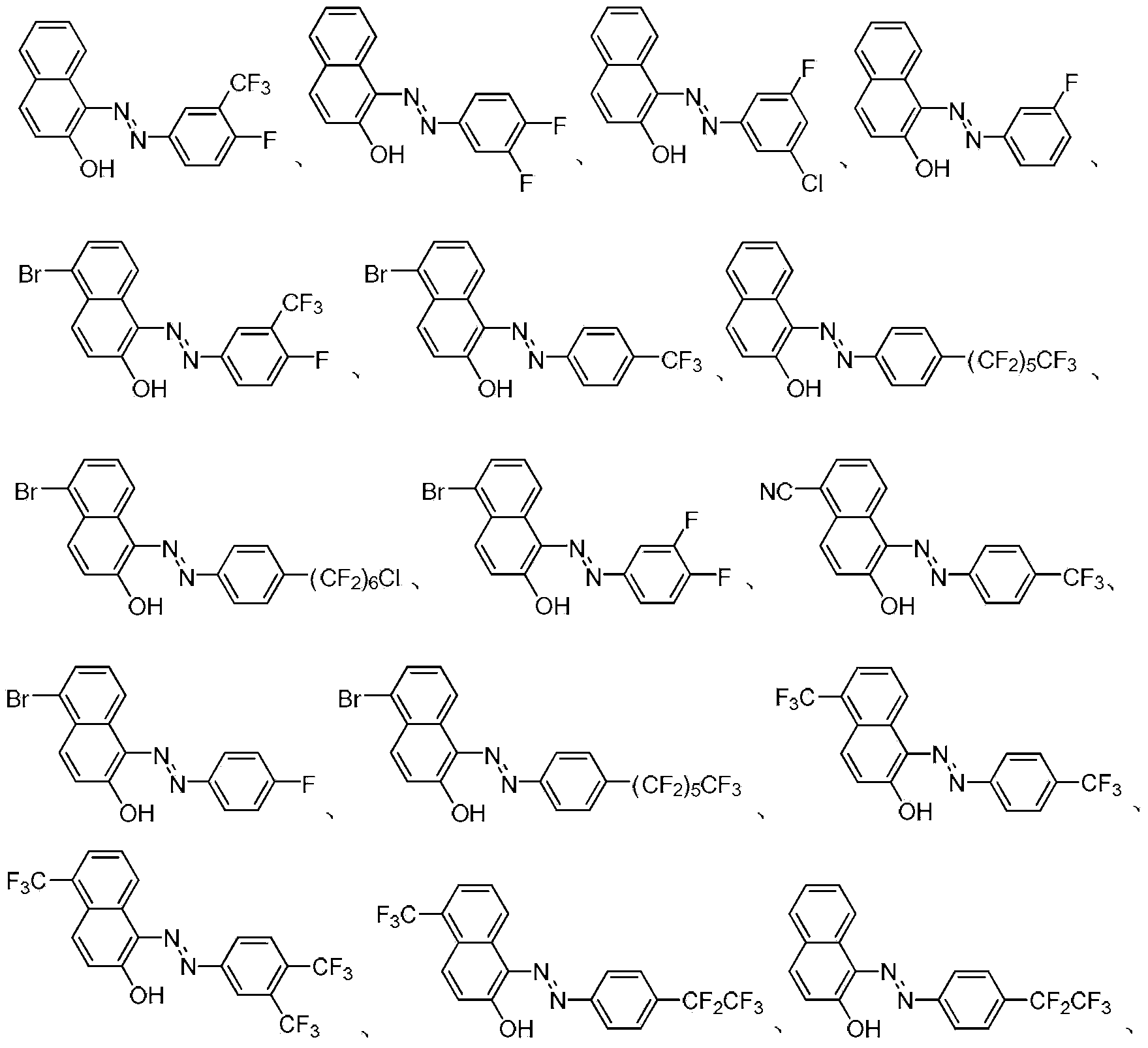
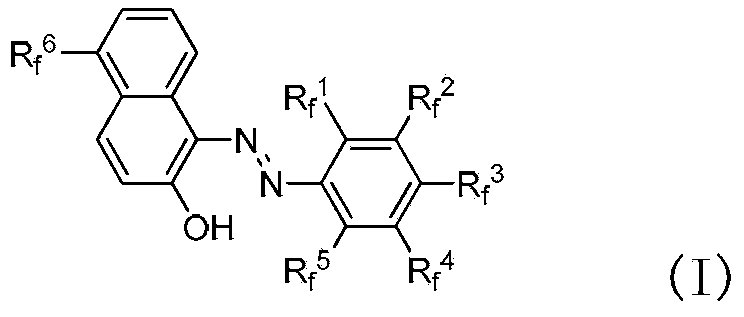
Fluorine-containing extractant and application thereof
The invention relates to a fluorine-containing extractant for lithium isotope separation and application thereof. Specifically, the invention discloses a fluorine-containing extractant for lithium isotope separation shown as formula (I) and an extraction organic phase containing the extractant. Being cheap and easily available, the extractant has the characteristics of excellent chemical stability, and easy back extraction and regeneration cycle. The extraction organic phase has a high extraction rate on lithium ions and is easy to gather 7Li, thereby realizing separation of the lithium isotope.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
System and method for isotope separation
InactiveUS20070272557A1Reduce choiceEasy to separateElectrostatic separatorsLiquid separation by electricityCapacitanceParallel plate
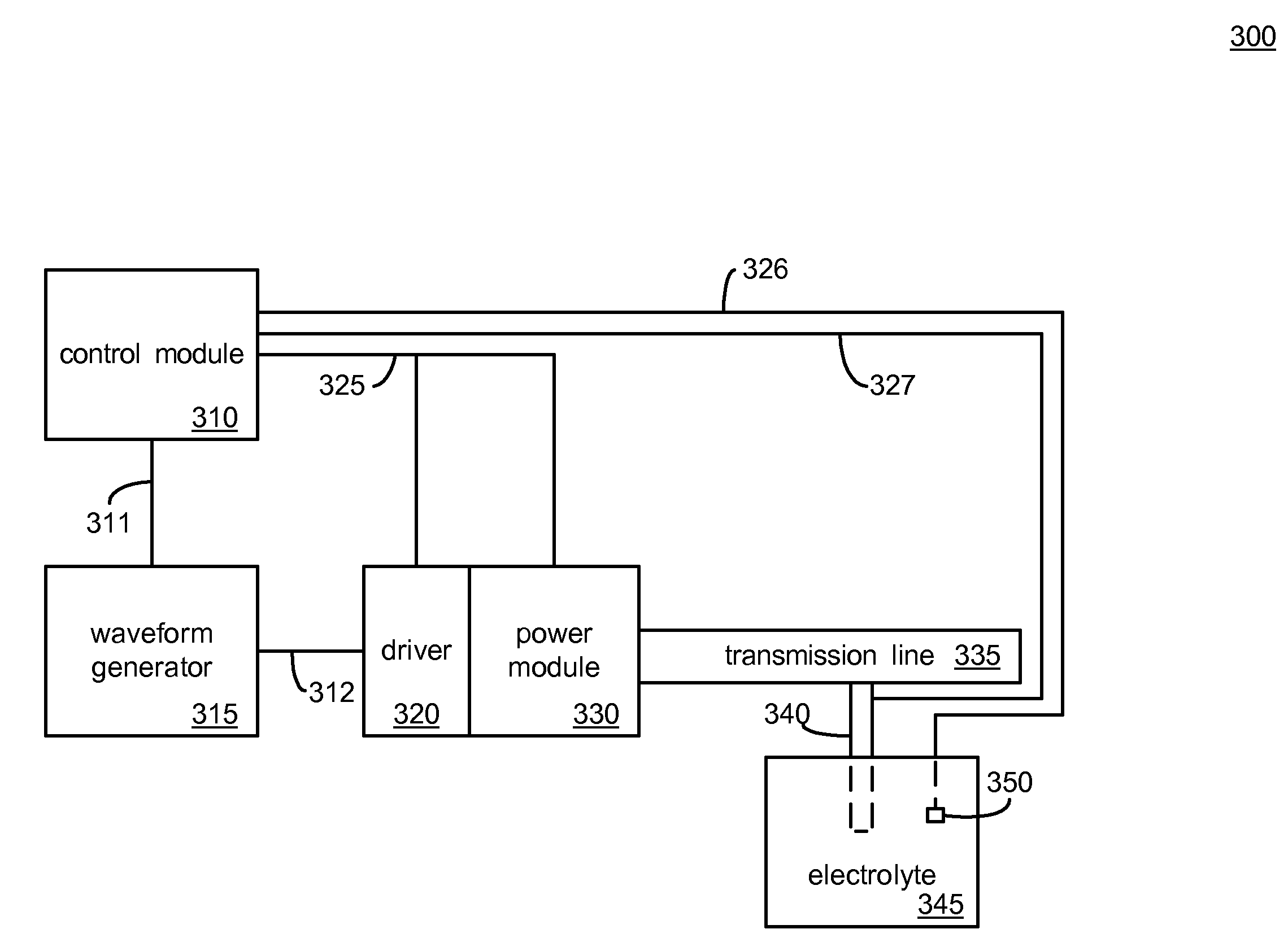
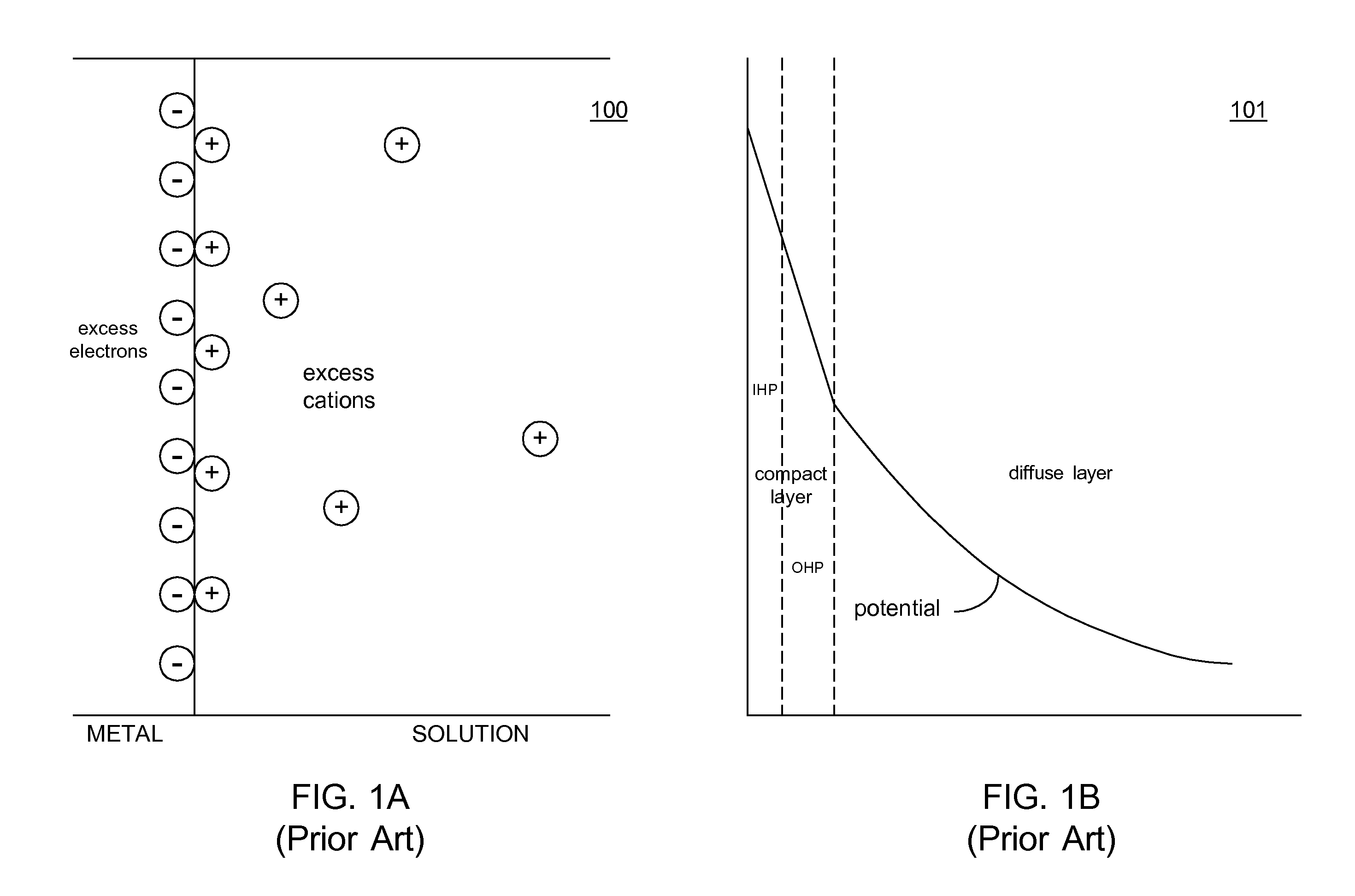
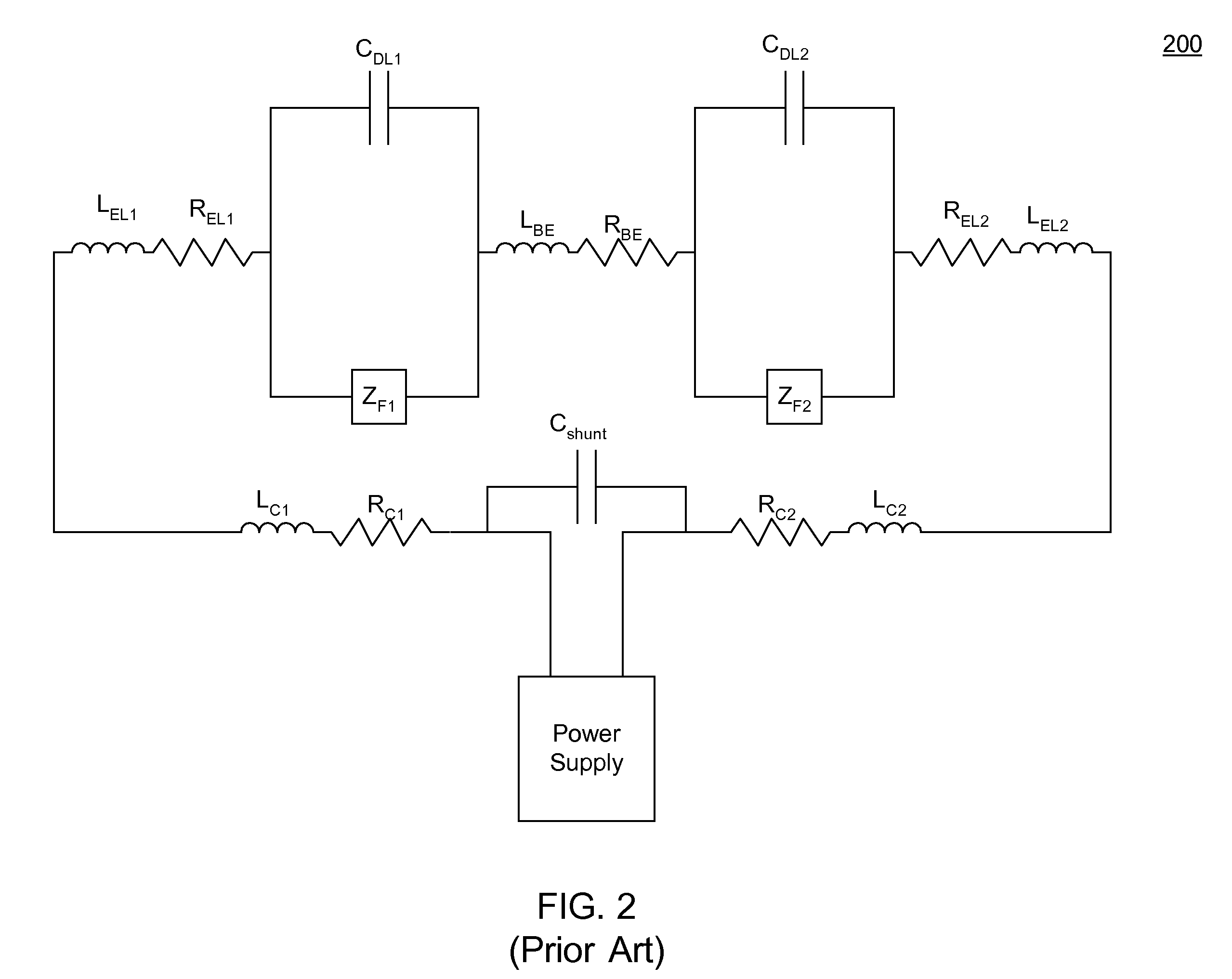
An anode and cathode for an electrolytic cell configured as a low inductance transmission line to enable control of an interphase at an electrode surface. The anode and cathode are coupled to a switched current source by a low inductance path that includes a parallel plate transmission line, a coaxial transmission line, or both. The switched current source provides fast switching between current sources to provide fast charging and discharging of the double-layer capacitance associated with the electrode surface so that an isotope may be selectively transported to the electrode surface for oxidation or reduction. A photon source may be used to create a population of isotope containing species within the electrolyte. An additional static magnetic field and / or an alternating current magnetic excitation source may be used to modify the composition of the population of species containing the isotope to be separated.
Owner:MATTHEWS MEHLIN DEAN
Method for removing water in anisole by using loaded type calcium oxide water removing agent
ActiveCN105294404AImprove water removal efficiencySolve bottlenecksEther separation/purificationCalcium hydroxideChemical reaction
The invention provides a method for removing water in anisole by using a loaded type calcium oxide water removing agent and relates to an industrial anisole water removing method. The method is characterized by comprising the following steps: filling the loaded type calcium oxide water removing agent into a fixed-bed reactor and pumping anisole liquid containing trace amount of water by using a high-pressure pump; controlling a reaction temperature to be 25 to 160 DEG C to react; by using a CaO+H2O=Ca(OH)2 chemical reaction, finally obtaining an anhydrous anisole solution, wherein the content of the anisole in raw materials is 70 percent to 99 percent and the mass percent of calcium oxide in the loaded type calcium oxide water removing agent is 5 percent to 30 percent. With the adoption of the method, a bottleneck problem of the trace amount of water in the anisole to a boron isotope separation industrial production process is solved; meanwhile, influences that a pasty calcium hydroxide product generated on the outer surface in a calcium oxide water removing process covers calcium oxide and a water removing reaction is not continuously carried out are avoided, and the calcium oxide water removing efficiency is improved.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Rotary low-temperature hydrogen isotope separation system and separation method thereof
The invention discloses a rotary low-temperature hydrogen isotope separation system and a separation method thereof, which solve the complicated construction, complicated parameter control, and high operation cost, or helium gas supply requirement, hydrogen and helium separation, and complex separation column regeneration treatment, or high cost of palladium displacement chromatogram filling material and easy failure in the prior art. The separation system includes a cryogenic tank, a reversing frame, a separation column, a raw material hydrogen-storage tank, a light isotope storage tank, a heavy isotope storage tank, a vacuum pump, and an exhaust gas storage tank. The separation method is characterized in that a separation column absorbing the raw material hydrogen to gradually separate the liquid nitrogen liquid level to desorb the hydrogen isotope to complete rearrangement of hydrogen isotope in other separation columns; and the light and heavy isotopes can be finally collected respectively from the far and near ends of the loop. The system creatively utilizes the technical principle of a simulated moving bed, the hydrogen isotope can continuously circulate in the closed loop separation loop, the heavy isotope concentration gradually decreases from the near end to the far end where the desorption occurs in the separation loop, and the concentration gradient of the heavy isotope is continuously increased with the adsorption / desorption frequency.
Owner:MATERIAL INST OF CHINA ACADEMY OF ENG PHYSICS
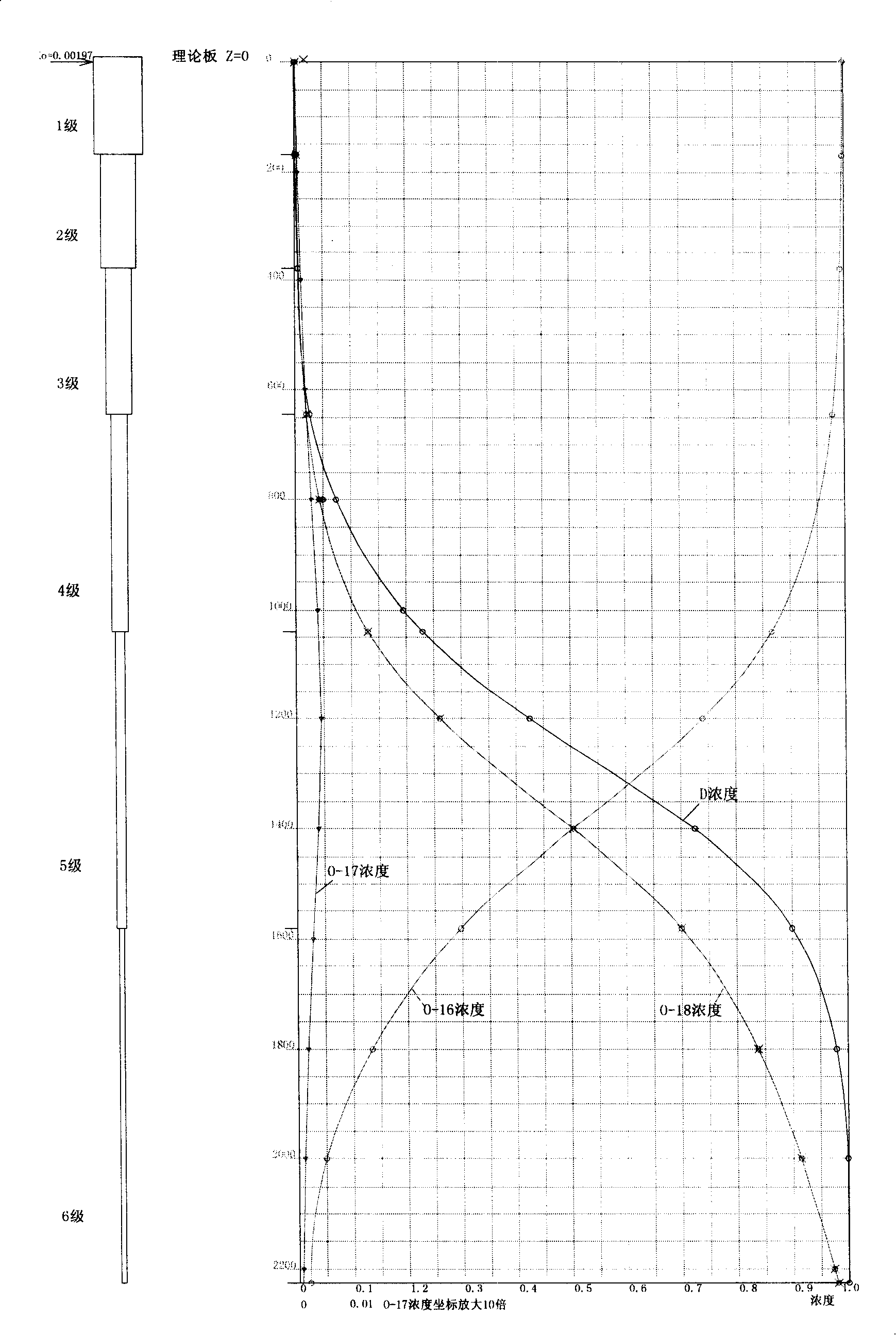
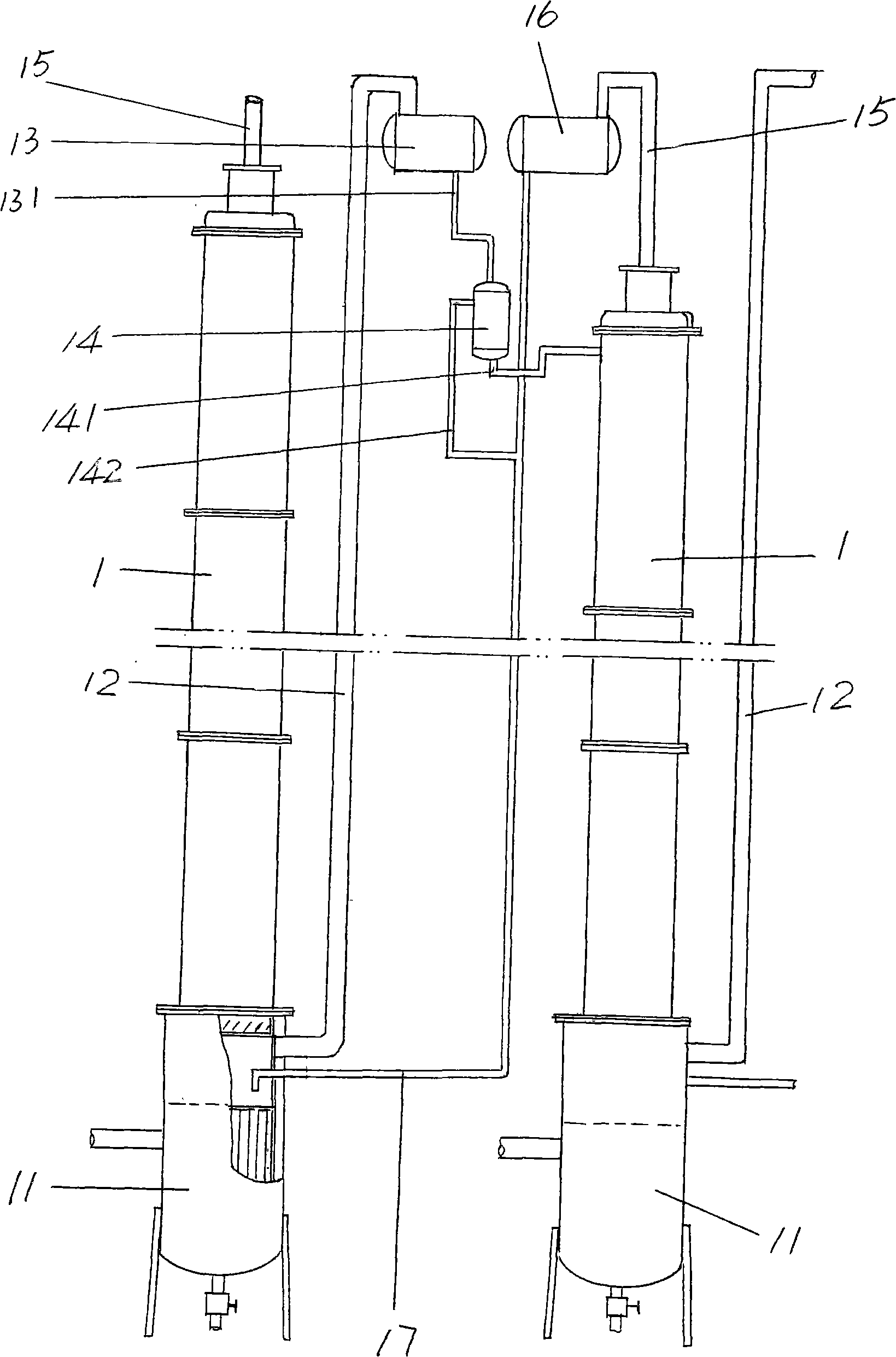
Method for producing doubly labelled water by water distillation and distillation equipment for enrichment production of doubly labelled water
The invention relates to a production method and a production device of chemical products, in particular to the production method of doubly labeled water consisting of deuterium and heavy oxygen O<18> enriched by a water reification method as well as a water rectification device thereof. The rectification device provided by the invention consists of a rectification tower in multilevel series or in multilevel and parallel series; each level of the rectification tower are connected in phase transition way; the connection way is to transport the steam in tower bottom to the top of the lower tower by using pressure difference and the steam is condensed to be liquid phase to enter lower level tower; ascending steam of the lower tower top is condensed to be liquid phase and then flows into higher level tower top automatically. In the invention, as a reboiler at the tower bottom adopts falling film evaporation, the tower bottom is dry all the time without the retention of liquid to greatly save the balancing time. On the other hand, as no rotating parts such as a pump, etc. is adopted, the possible troubles caused by leakage, power failure, mechanical fault, etc. can be avoided to ensure constant and reliable operation of the system, which is important for isotope separation.
Owner:JIANGSU HUAYI TECH
Construction method based on technology for producing boron isotope by anisole-boron trifluoride
ActiveCN104190256AImprove separation efficiencyReduce the amount requiredIsotope separationBoron halogen compoundsBoron trifluorideProduct gas
The invention discloses a construction method based on a technology for producing boron isotope by anisole-boron trifluoride, which belongs to the field of isotope separation, and mainly aims at solving the problems such as low yield of boron-10 and incapability of obtaining qualified boron-11 products at the same time in the existing construction method based on the technology for producing boron isotope by anisole-boron trifluoride. The method disclosed by the invention comprises the following steps: continuously purifying raw materials, namely boron trifluoride gas and an anisole liquid, as well as an anisole-boron trifluoride complex before entering a cracking tower, nitrogen gas coming out of the top of a complex gas stripping tower, boron trifluoride gas after cracking, and gas coming out of the top of a first exchange rectifying tower; adding the purified nitrogen gas into a second exchange rectifying tower; adding fresh boron trifluoride gas into the first exchange rectifying tower, wherein boron-10 is not discharged out from the top of the cracking tower and boron-11 is not discharged out from the top of a complexation tower; and after performing system rectification balance for 72-148 hours, converting to the normal production mode. The method has the advantage that the two qualified products, namely boron trifluoride-10 and boron trifluoride-11 can be produced at the same time.
Owner:浙江创世雷博科技有限公司
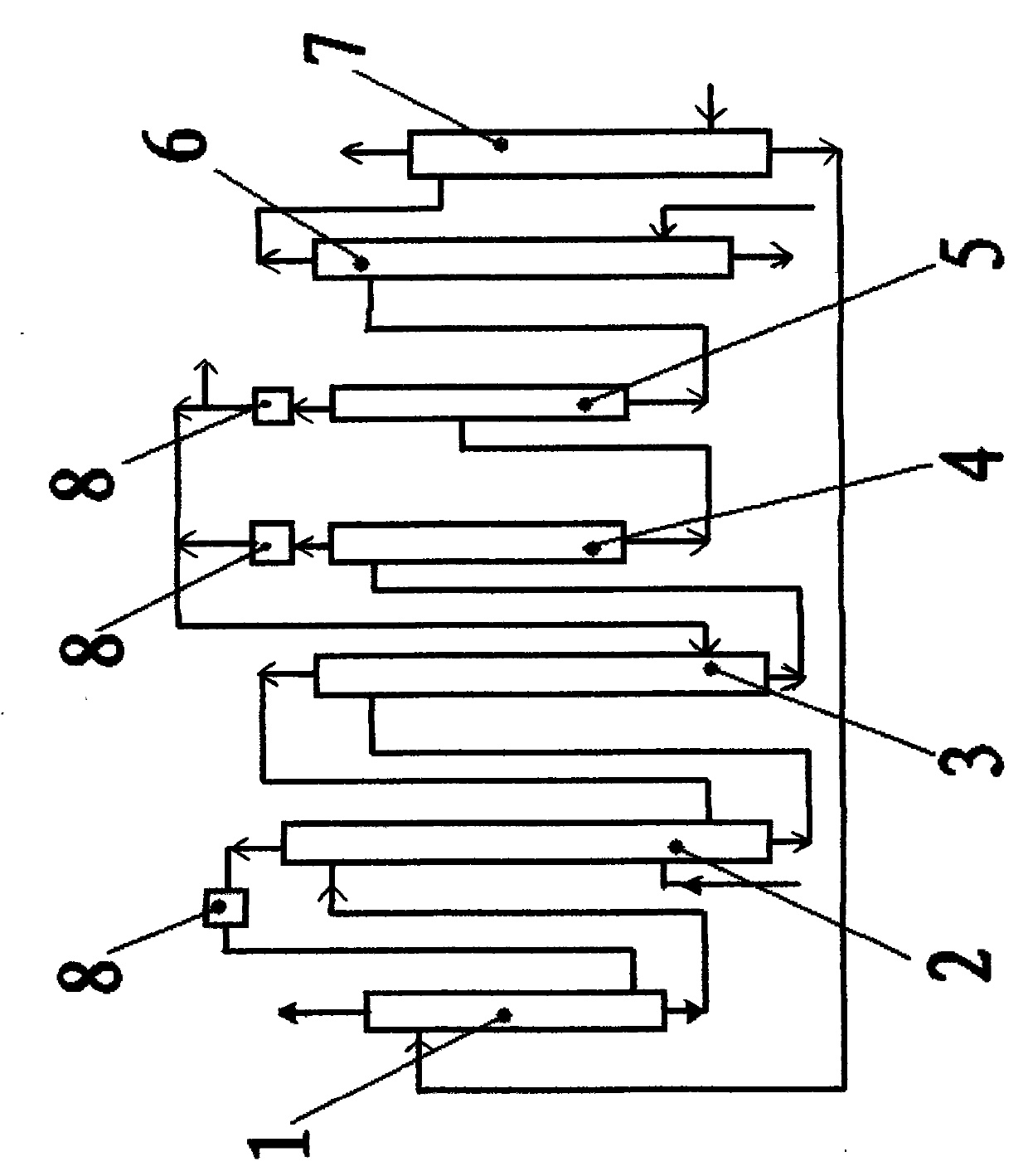
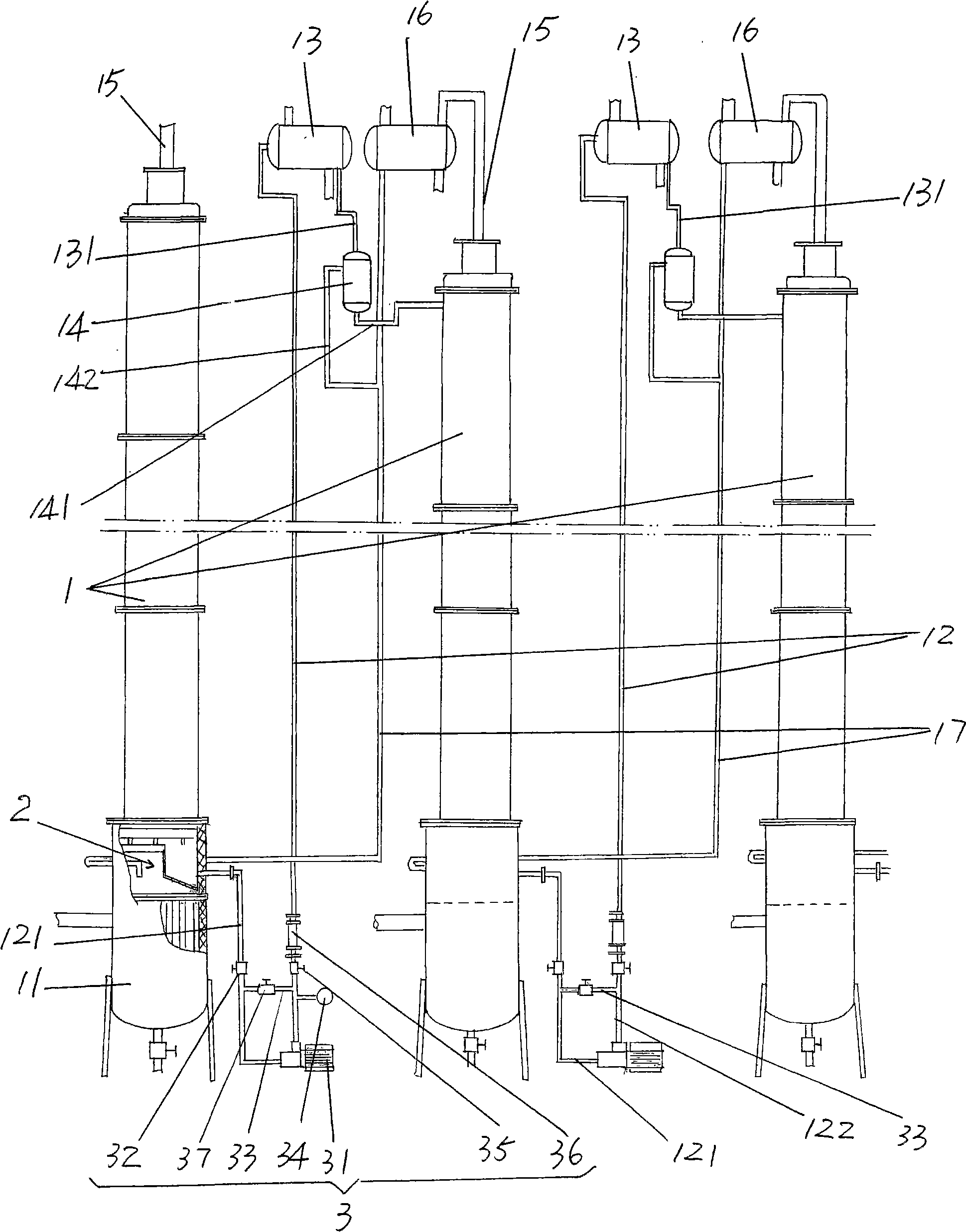
Liquid phase cascade rectification device for isotope separation
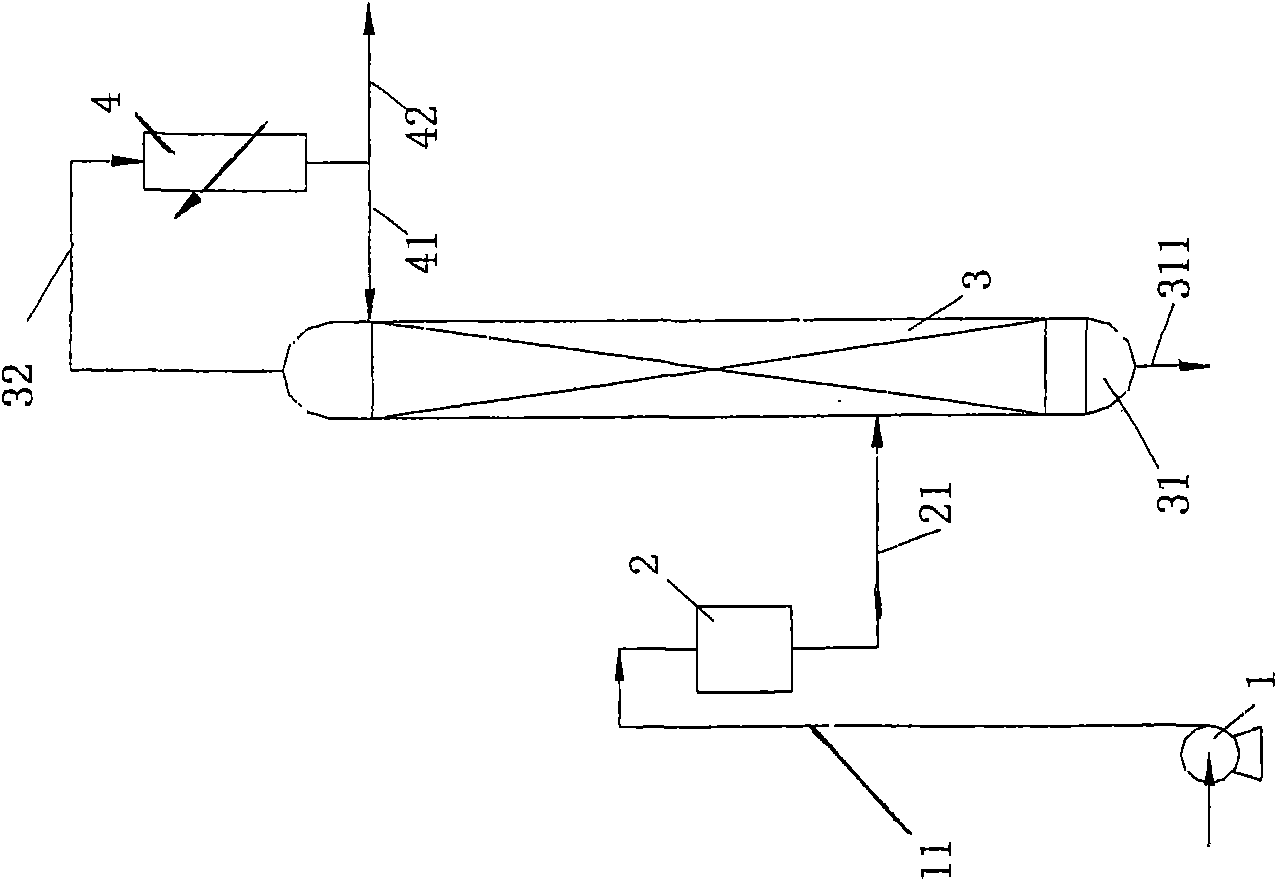
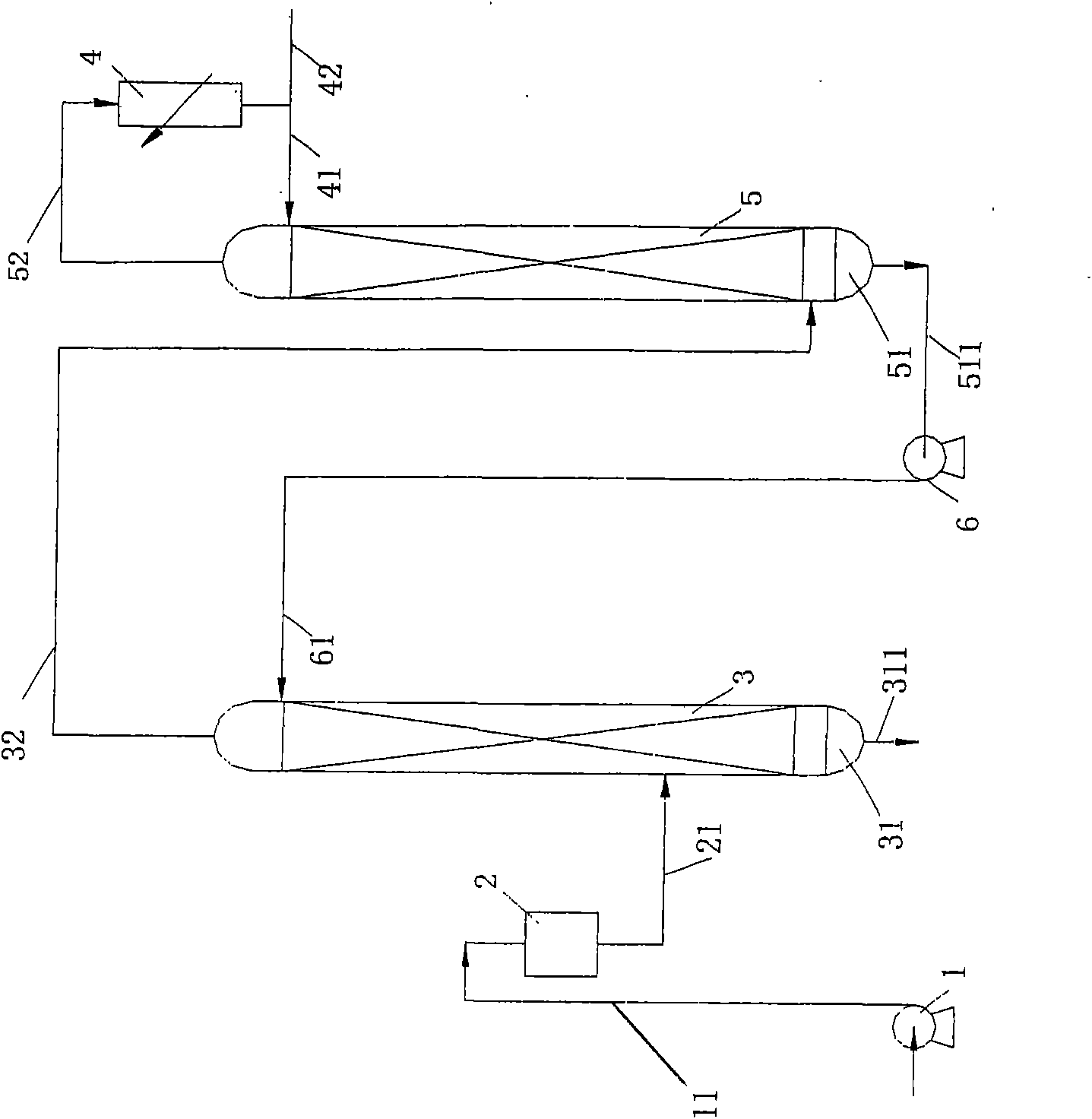
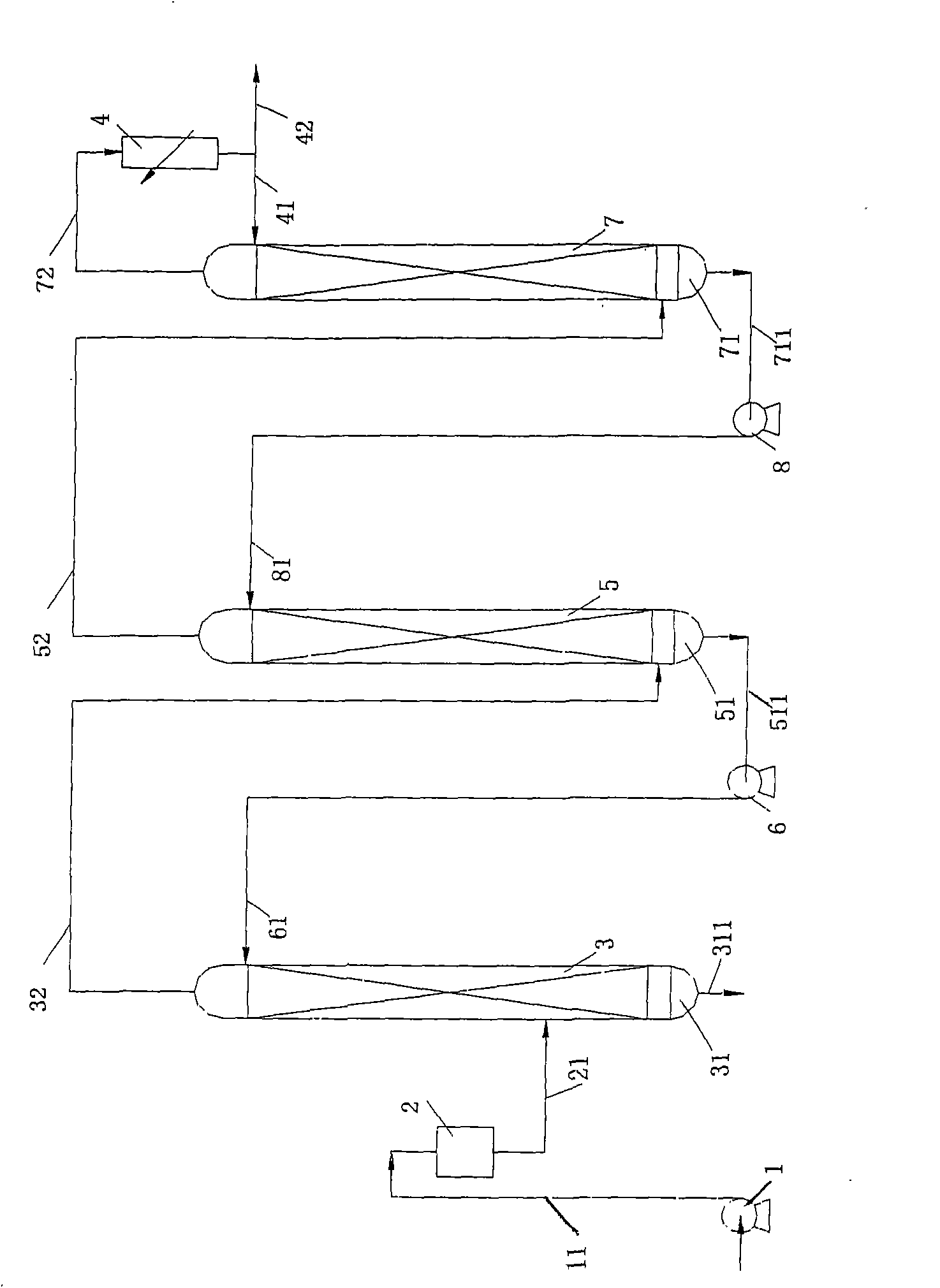
ActiveCN101298025AAvoid backmixingAvoid the reboil processIsotope separationFractional distillationRefluxReboiler
The invention discloses a liquid phase cascade rectifying device for isotope separation, which pertains to the technical field of separation and rectification equipment. The rectifying device comprises a group of rectifying towers which are mutually connected through pipes and each rectifying tower comprises a bottom reboiler, a first pipe, a first overhead condenser, a second overhead condenser, a liquid storage tank, a vapor pipe and a reflux pipe. The rectifying device is characterized in that: the first pipe is provided with a liquid circulating mechanism which is applied to leading liquid from a previous stage of rectifying tower to the top of a next stage of rectifying tower; a catch mechanism which is used for separating the liquid which is in the next stage of rectifying tower and led into by the reflux pipe from the liquid fell from the previous stage of rectifying tower is arranged at the lower part of the rectifying tower and the corresponding upper part of the bottom reboiler. The device has the advantages of being conducive to saving energy and guaranteeing separation efficiency.
Owner:JIANGSU HUAYI TECH
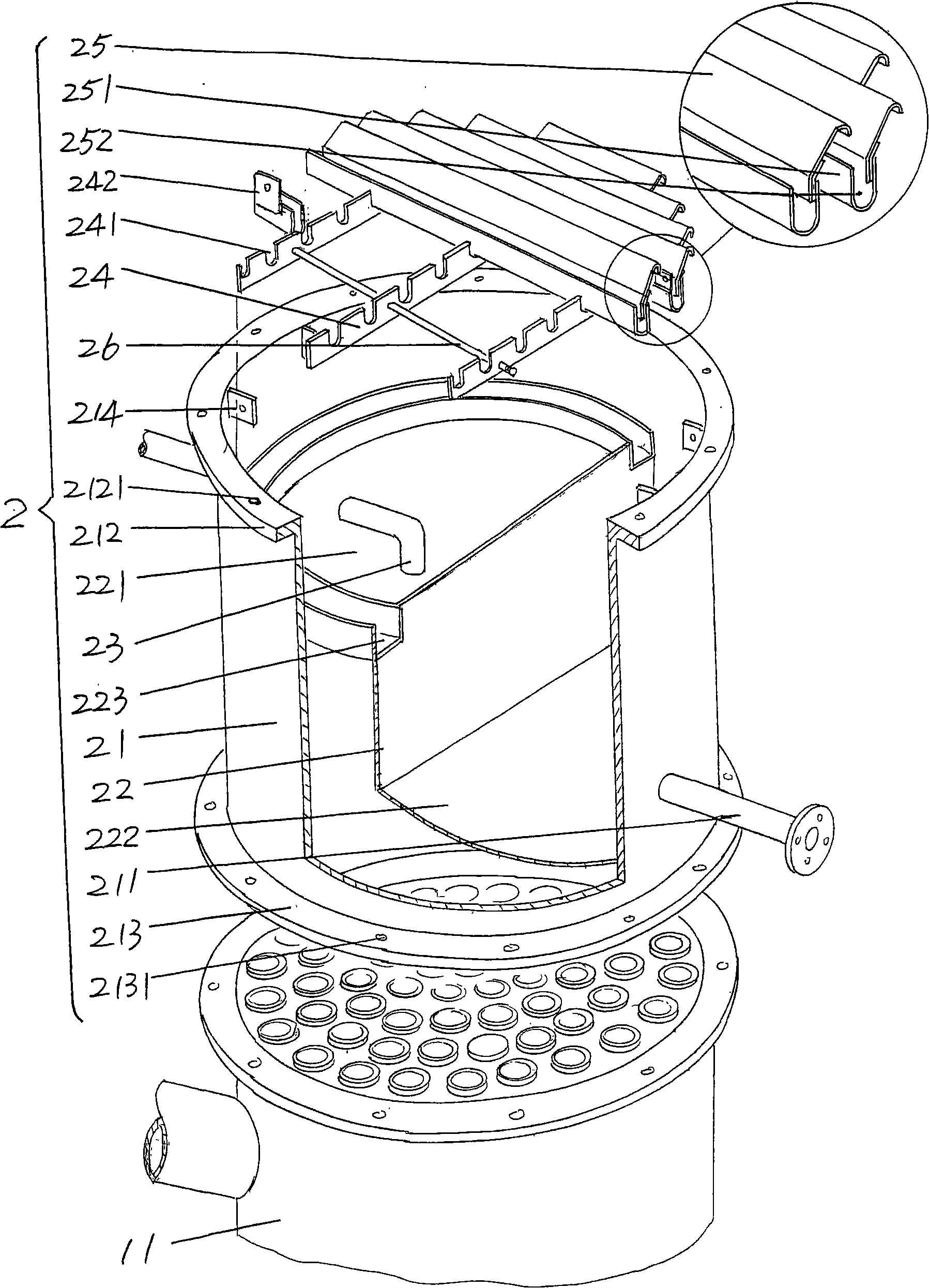
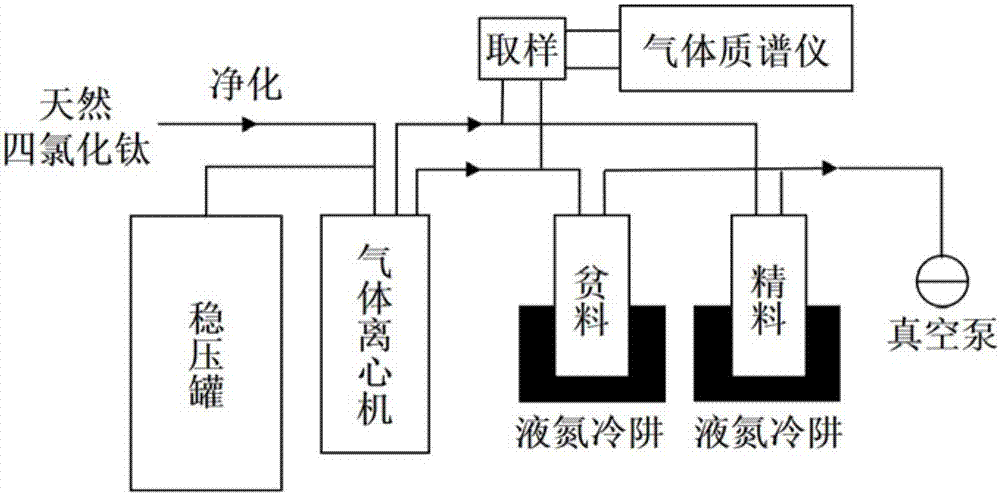
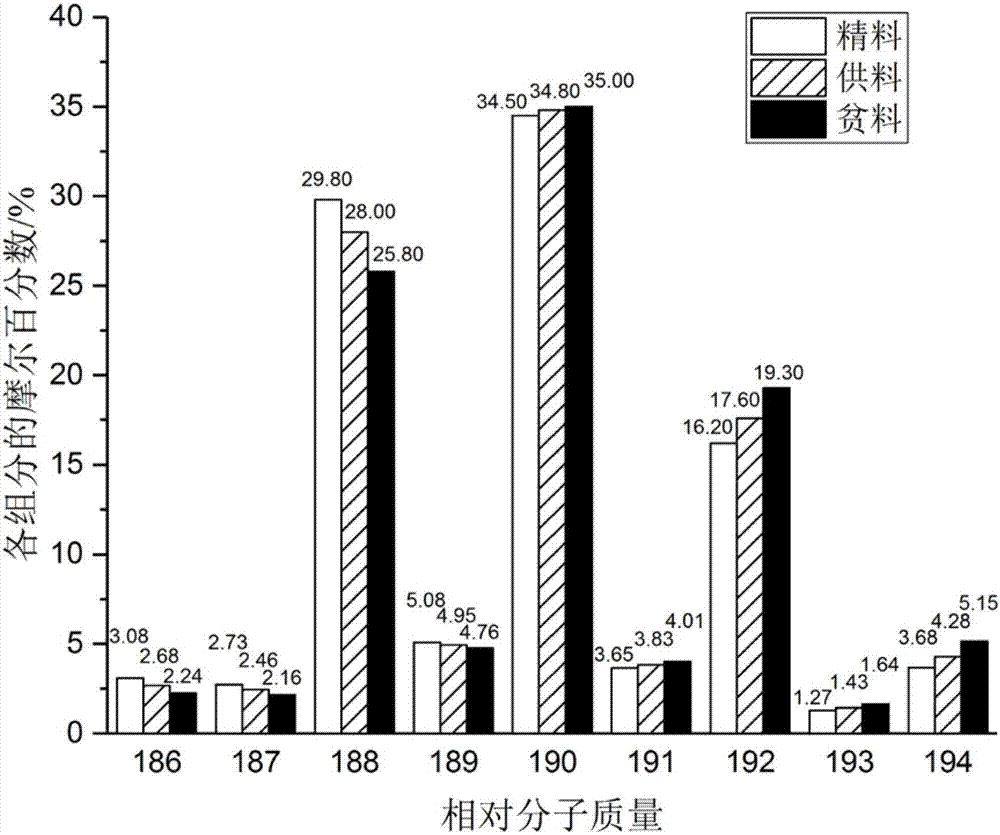
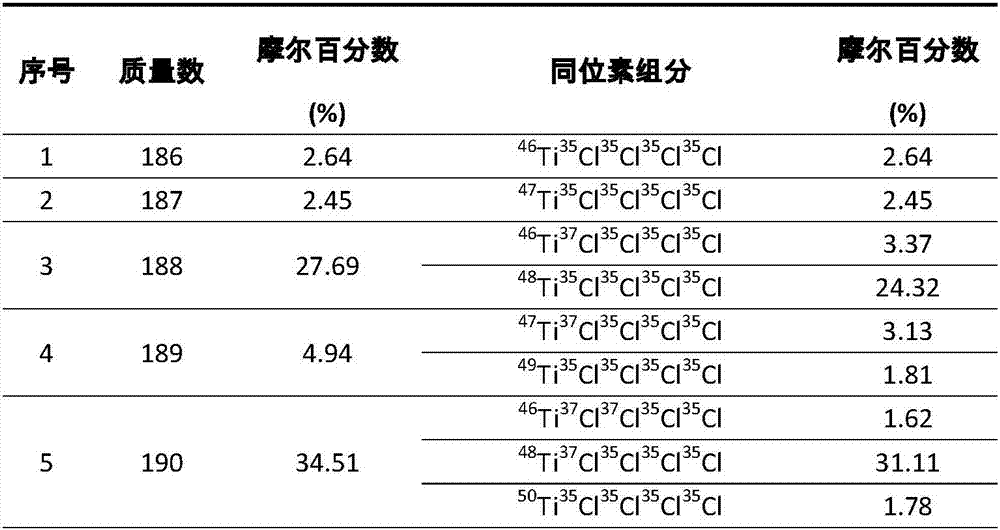
Centrifugal separation method of titanium isotopes
InactiveCN107441933AReduce energy consumptionHigh separation factorIsotope separationSeparation coefficientGas centrifuge
The invention discloses a centrifugal separation method of titanium isotopes. The centrifugal separation method specifically comprises the step of separating the titanium isotopes by virtue of a domestic gas centrifuge and a centrifugal separation working medium titanium tetrachloride. The centrifugal separation method is characterized by comprising the steps of introducing purified gas-state titanium tetrachloride into the domestic gas centrifuge in a certain feeding flow, adjusting the pressure intensity of a feeing pipe hole of the centrifuge and pressure intensity parameters of concentrated and lean pipe holes, respectively receiving the material at concentrated and lean ends, carrying out sampling when each parameter is stabilized, and carrying out analysis by virtue of a gas mass spectrometer so as to realize the separation of the titanium isotopes and evolution of a separation effect. The method is low in energy consumption, relatively large in separation coefficients and suitable for cascading large-scale production; and furthermore, the obtained lean material and the raw material are only different from abundance, and the obtained lean material can be recycled as a chemical reagent to be continuously used, so that the centrifugal separation method of the titanium isotopes has certain advantages.
Owner:TSINGHUA UNIV
Industrial production method for separating boron isotope product based on methyl-phenoxide-boron trifluoride complex
ActiveCN104209003AAvoid cloggingLess side effectsIsotope separationBoron halogen compoundsChemical reactionBoron trifluoride
The invention discloses an industrial production method for separating a boron isotope product based on a methyl-phenoxide-boron trifluoride complex, belonging to the technical field of separation of isotope. The industrial production method mainly aims to solve the problem that a methyl-phenoxide-boron trifluoride complex chemical reaction exchange rectifying method is serious during vacuum material carrying and the like. The industrial production method comprises the following steps: firstly, performing gas stripping and drying treatment to the methyl-phenoxide-boron trifluoride complex, continuously adding the methyl-phenoxide-boron trifluoride complex from a gas stripping tower kettle into a first exchange rectifying tower kettle, continuously extracting liquid in the fore exchange rectifying tower kettle into the next tower top, condensing steam at the top of the next tower top, and continuously entering the fore tower kettle; under pressure-reduced heating distillation, enabling a boron trifluoride gas and methyl ether gas to enter shell passes of a condenser and a catcher, enabling a coolant to travel the shell passes of the condenser and the catcher, wherein a minus 60DEG C methyl ether solution pump is conveyed to a spray device so as to spray a boron trifluoride gas, the boron trifluoride gas and the methyl ether gas are converted into the methyl-phenoxide-boron trifluoride complex. The industrial production method has the advantage of being capable of solving the problem of vacuum material carrying.
Owner:浙江创世雷博科技有限公司
Process and apparatus for condensation repressing isotope separation by laser activation
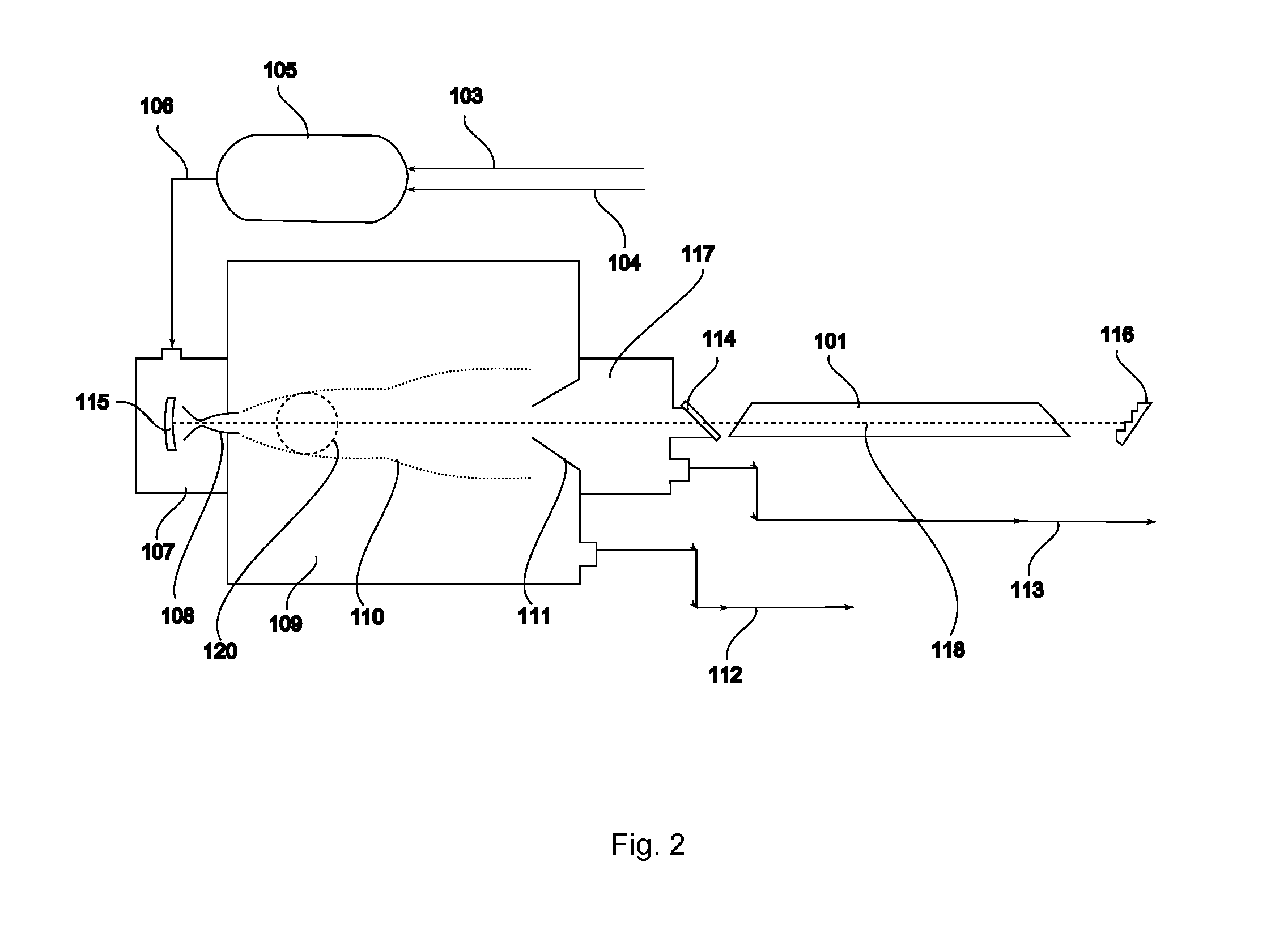
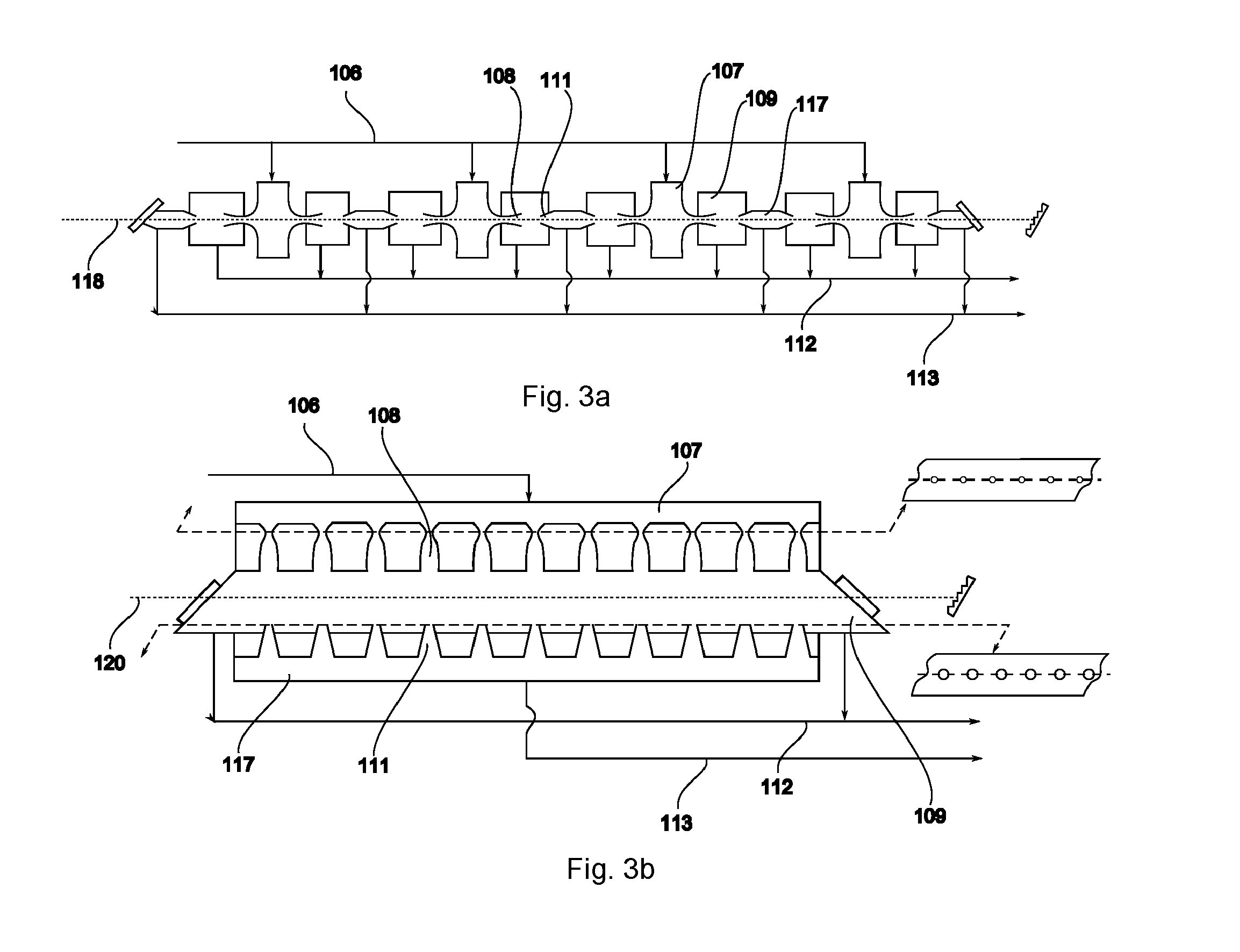
ActiveUS20140270035A1Low costAvoid formingConversion outside reactor/acceleratorsIsotope separationBiological activationLaser beams
Isotope enrichment by laser activation wherein a multi-isotopic element Q, like Uranium, Silicon, Carbon is incorporated into gaseous QFn, QF6, QF4, QOmFn, etc and diluted in gas G like He, N2, Ar, Xe, SF6 or other inert gas; and wherein that mixture is cooled by adiabatic expansion or other means encouraging formation of dimers QF6:G in a supersonic super-cooled free jet; and wherein that jet is exposed to laser photons at wavelengths that selectively excite predetermined molecules iQF6 to iQF6*, thereby inducing rapid VT conversions and dissociations of iQF6*:G→iQF6+G+kT, while leaving non-excited dimers jQF6:G intact; and wherein a skimmer separates the supersonic free-jet core stream containing heavier jQF6:G dimers from lighter core-escaped iQF6-enriched rim gases. Particularly an advanced technique is disclosed to enrich iUF6 by free jet expansion and isotope-selective dimerization suppression, utilizing a molecular CO laser and intra-cavity UF6 irradiation with laser lines overlapping predetermined iUF6 absorptions; and providing multiple free jet separator units irradiated by one laser beam, thereby enhancing process economics.
Owner:CRISLA INC
Czochralski growing method of magnesium aluminate spinel crystal doped with transition metal ions
ActiveCN104005088ASmall sizeQuality improvementPolycrystalline material growthBy pulling from meltIridiumSeed crystal
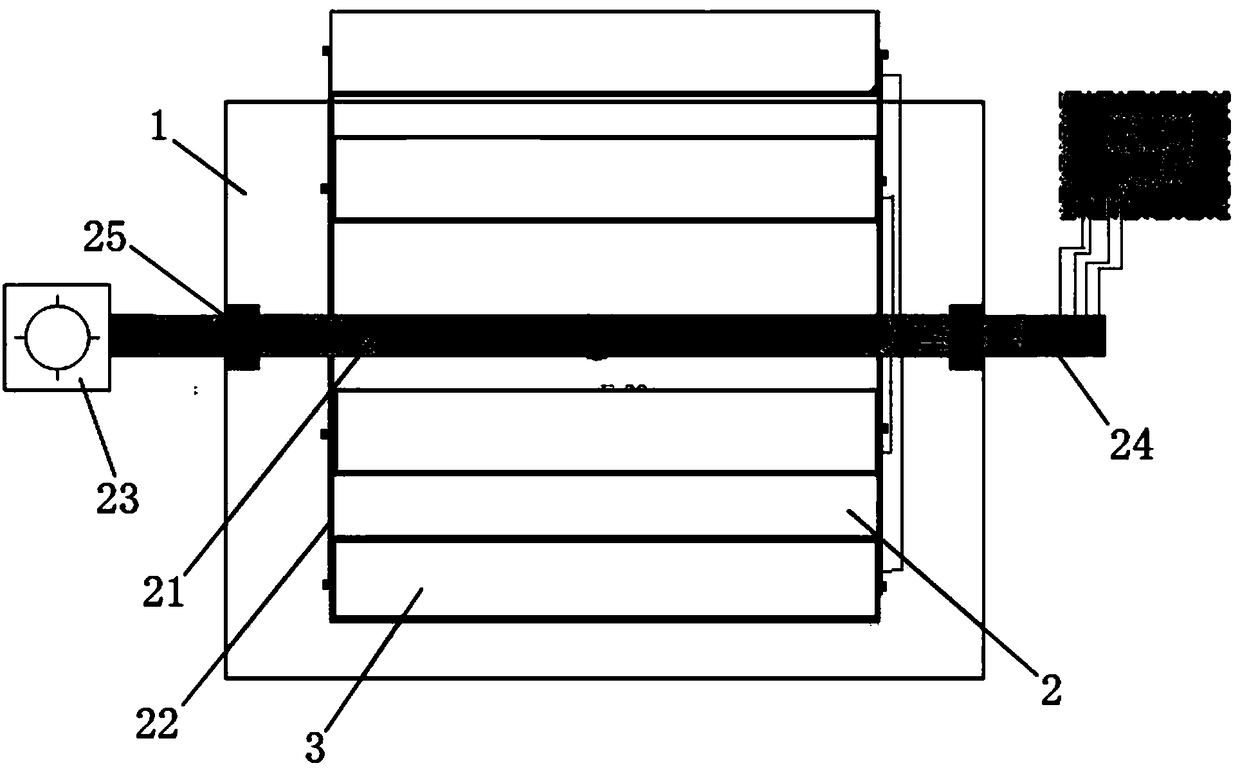
The invention discloses a growing method of a magnesium aluminate spinel crystal doped with transition metal ions. The molecular formula of the crystal can be expressed as TM2xMgAl2(1-x)O4 and Tm'yMg1-yAl2O4 (TM=Ti<3+>, Cr<3+>, Fe<3+> and Ni<3+>; TM'=Mn<2+>, V<2+> and Co<2+>, 0<x<1, and 0<y<1). The growing method is characterized in that MgAl2O4 polycrystalline raw materials synthesized with the flame method and transition metal oxides prepared according to the proportional concentration are placed in an iridium crucible and sufficiently heated to be molten, the magnesium aluminate spinel crystal or the doped magnesium aluminate spinel crystal is adopted as a seed crystal, and crystal growth is conducted with the Czochralski method. According to the method, the crystal with the large size and high quality can be obtained and be expected to be applied to the fields of micromachining, laser medicine, laser chemistry, laser printing, military application, underwater communication and axis isotope separation.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
Atomic forcipes and nuclear magnetic isotope separation method and apparatus
InactiveUS20190162798A1Increase the rate of chemical reactionsPromote nuclear magnetic isotope ion intercalationGrapheneIsotope separationChemical reactionBiological activation
Atomic forcipes is a nanomechanical magnetoelectric element having an insulator, an atom-thick conductive graphene sheet suspended as a heterostructure onto the insulator, and a gallery between the insulator and the graphene sheet. Atomic forcipes can be actuated acoustically or electromagnetically. Activation generates a chemical potential of directionally enhanced chemical reaction rate. Atomic forcipes can be formed by selecting enhanced graphene having a particle size, providing piezoelectric smectite clay of the particle size, combining graphene particles with clay, adding a compatibilizer, and irradiating with ultrasound, UV, or microwaves. Isotope separation apparatus and methods are supported by atomic forcipes. A method by mixing an aqueous phase suspension of atomic forcipes with nuclear magnetic isotope (NMI) ions, applying ultrasound to promote NMI ion intercalation, applying ultraviolet light to generate free radicals on the NMI ions, and extracting enriched NMI ions from the piezoelectric sheets. Another method employs nuclear spin using nuclear magnetic stiction.
Owner:FULLLIVE INT LTD
Grain production place discriminating method utilizing heavy element isotope ratio composition
The present invention relates to a grain production place discriminating method which utilizes a heavy element isotope ratio composition. The isotope ratios of strontium and lead which are contained in the grain, namely an abundance ratio of a strontium isotope which is composed of mass numbers of 87 and 86, and a lead isotope which is composed of mass numbers of 208,207,206 and 204 are analyzed. The production place of the grain is discriminated through the analysis information.
Owner:一般财团法人日本谷物检定协会 +1
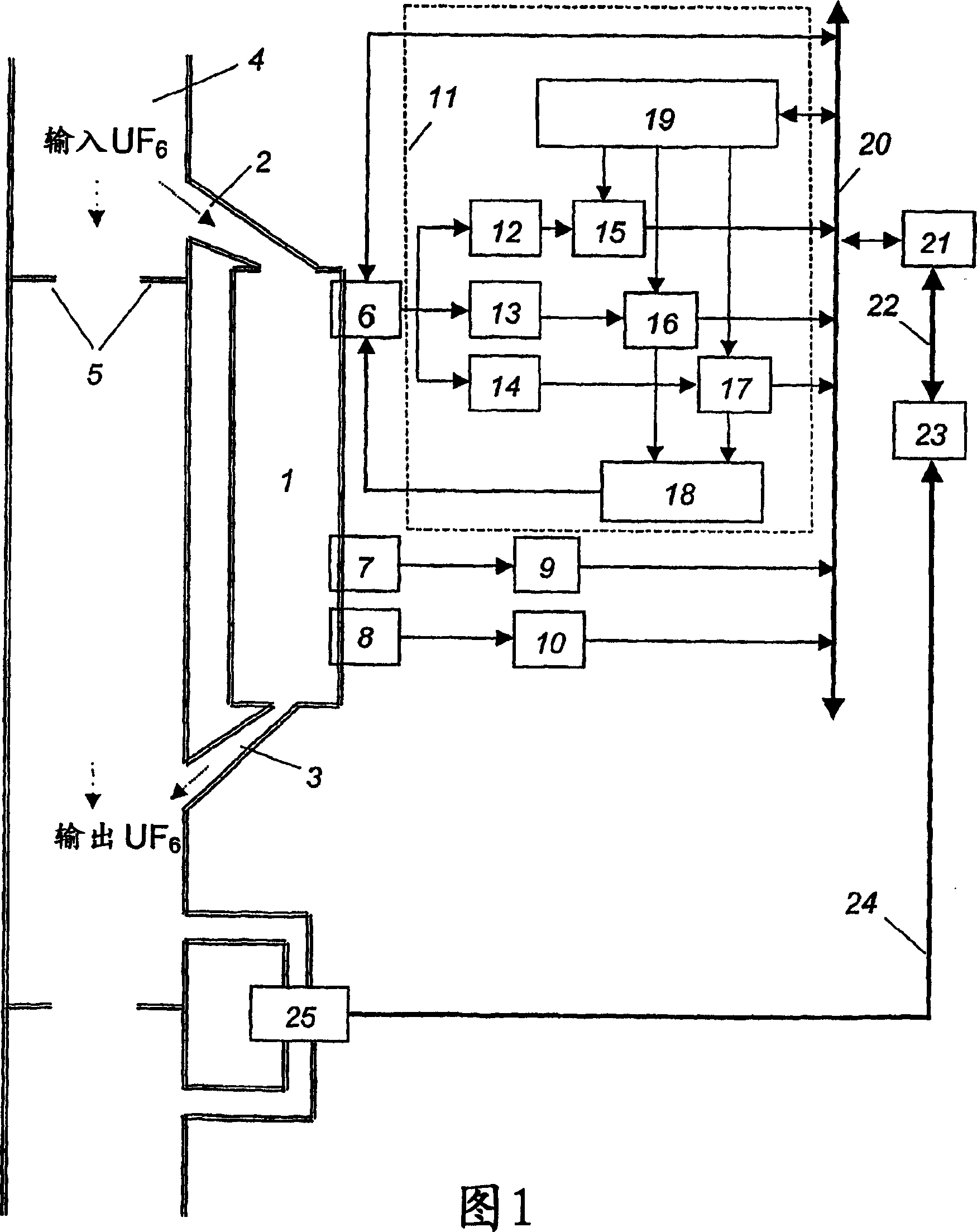
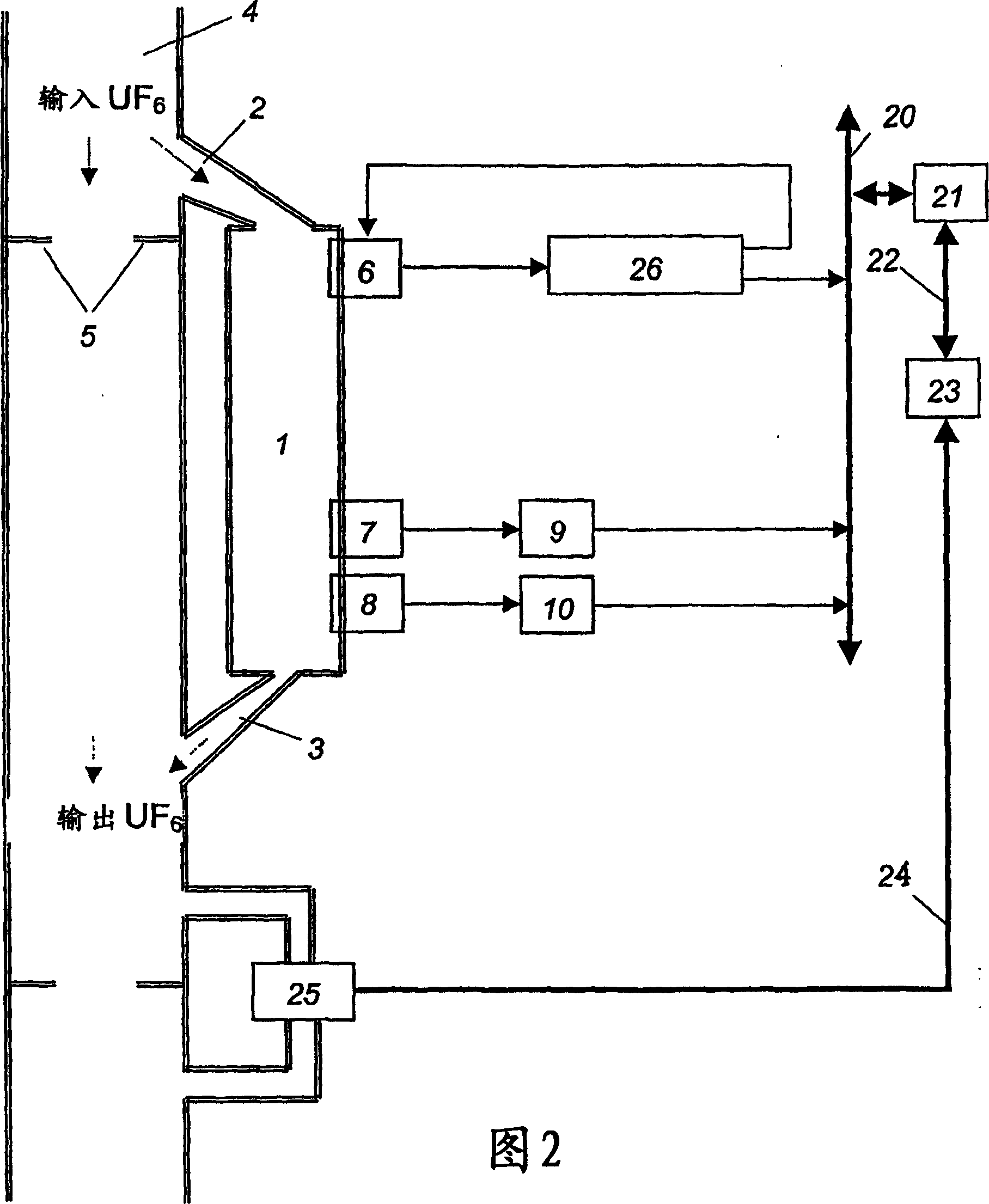
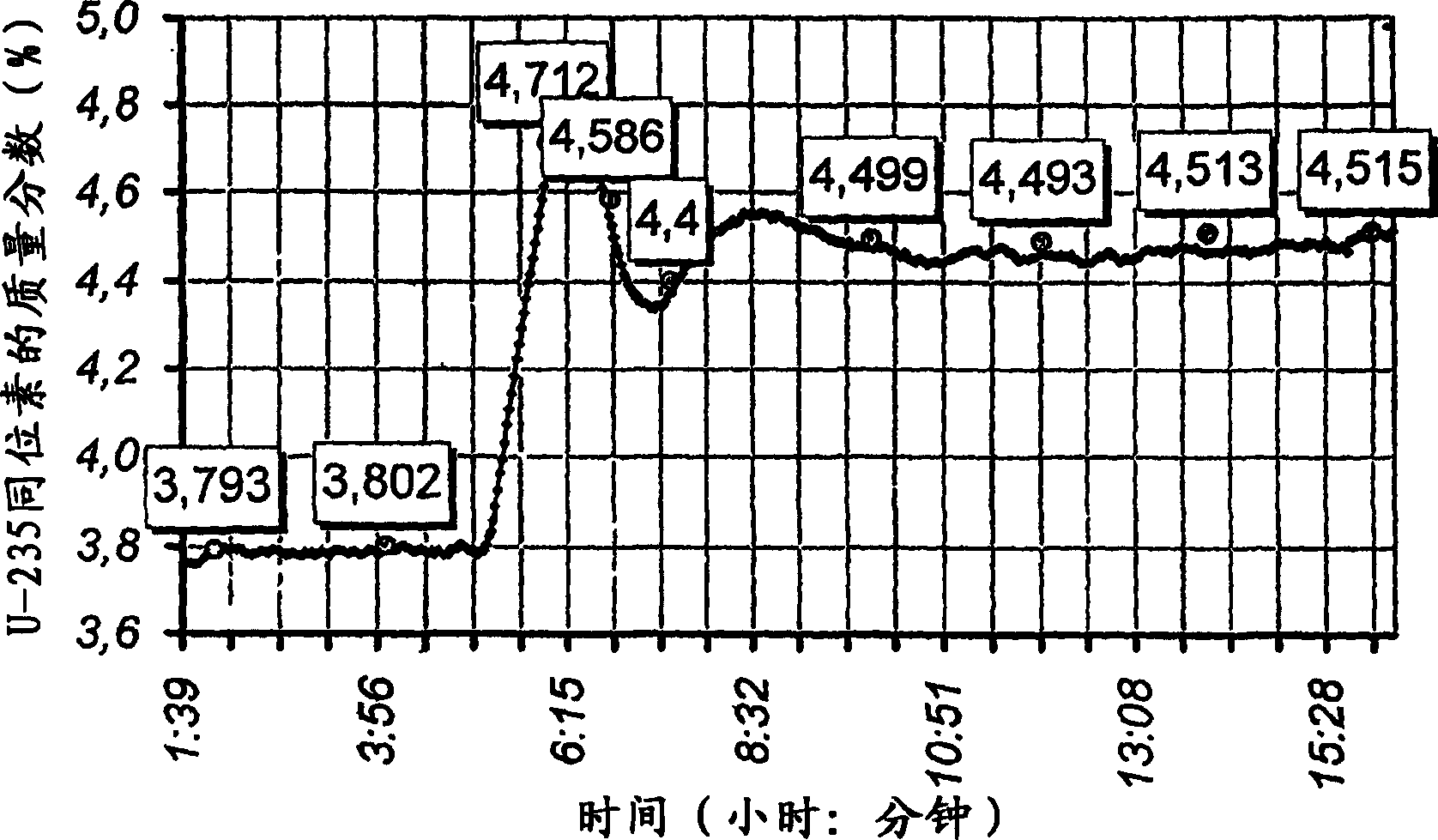
Method of controlling mass fraction of uranium-235 isotope in gas phase of uranium hexafluoride and measuring system for implementation of the method
InactiveCN1799106AAvoid inaccuraciesReduce accidental errorsNuclear monitoringMaterial analysis by transmitting radiationGas phaseUranium 235/Uranium 238
The invention relates to nuclear material analysis by radiation methods and can be used for on-line monitoring uranium hexafluoride enrichment in isotopic dividing production. The uranium-235 gamma-radiation intensity, the temperature and the pressure of uranium hexafluoride are determined by means of averaged measurement results within identical time intervals when a current time is changed at a value equal to the time segment of the time interval. The calculated value of the uranium-235 mass fraction is referred to the current time in coincidence with the measuring time interval. The invention also discloses a control system of the method, wherein at least three descriminators and a timer are mounted in a controller, the output end of the descriminator is connected with the input end of a gamma emission detector, each output end of the descriminators is connected with the input end of single electrical impulse counter.
Owner:ОТКРЫТОЕ АКЦИОНЕРНОЕ ОБЩЕСТВО «СИБИРСКИЙ ХИМИЧЕСКИЙ КОМБИНАТ»
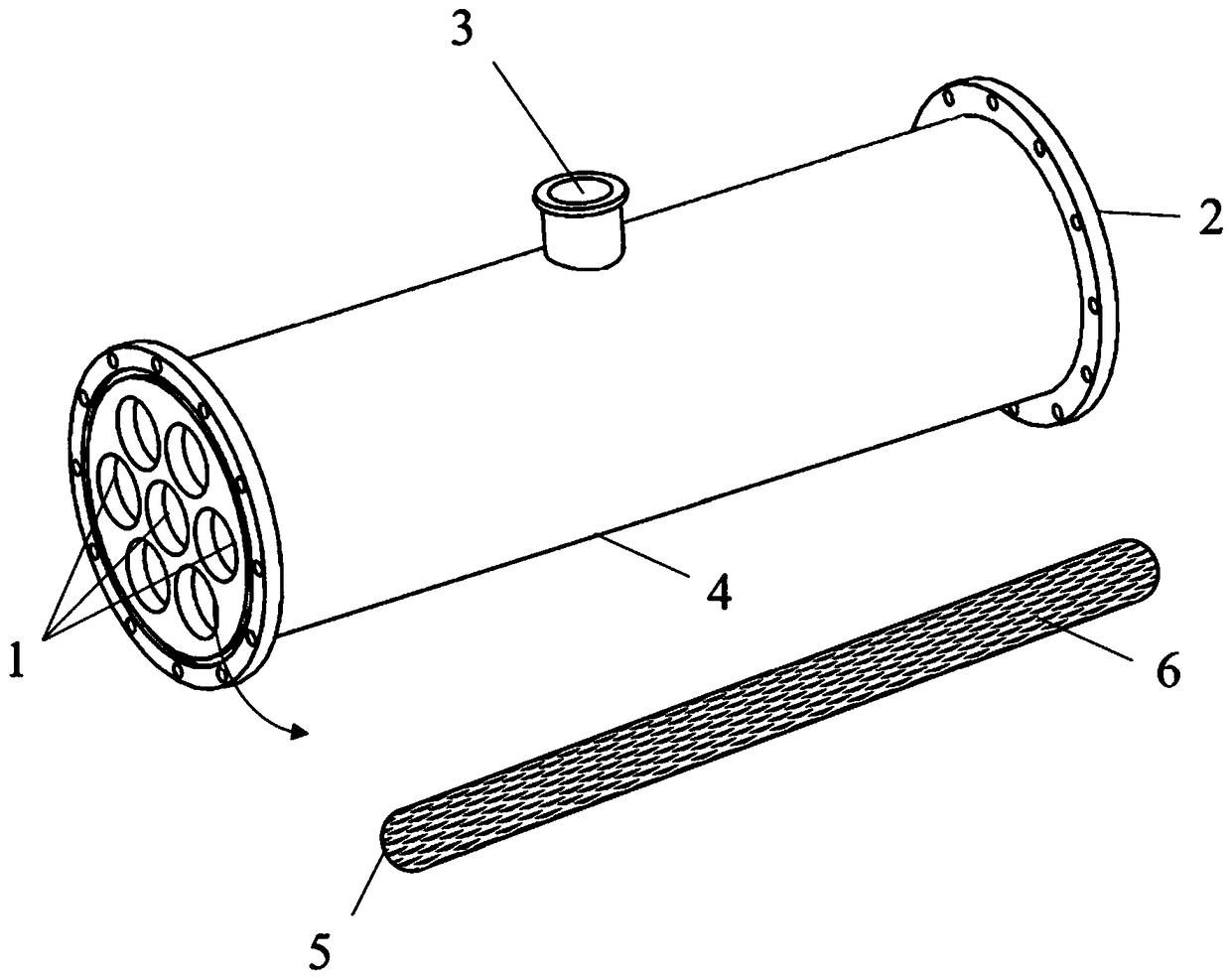
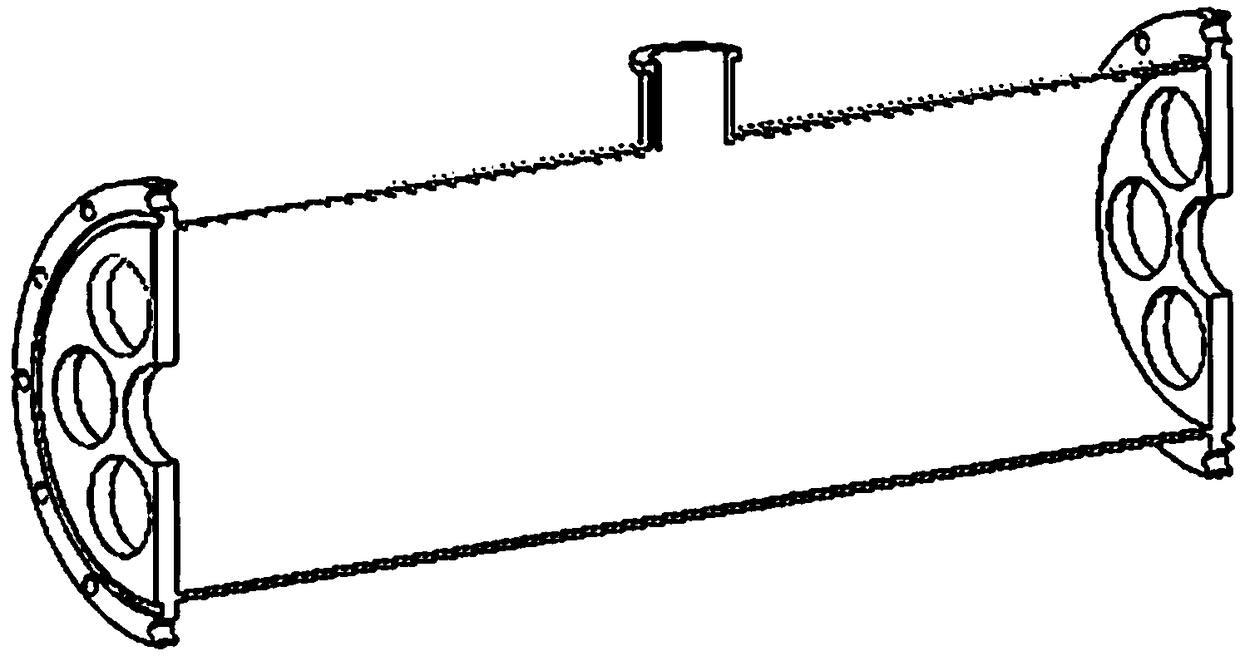
Membrane separation device and method for carbon isotope separation
PendingCN108939921AEfficient separationIncrease the effective ratioIsotope separationOutfallPorous membrane
The invention discloses a membrane separation device and method for carbon isotope separation. The device comprises a cylindrical cavity for loading a separation membrane tube, a stainless steel clothreel and a porous membrane (such as polyether sulfone resin or polypropylene screen), wherein the porous membrane winds and coats on the outer wall surface of the stainless steel cloth reel for exactly and completely coating by one circle; the side face of the reel is adhered and sealed with liquid glue; the reel coated with the porous membrane is loaded into the cylindrical cavity, and two endsof the reel are sealed with liquid glue; the cylindrical cavity with the separation membrane tube is provided with three openings, two openings are arranged at two axial ends, and one opening is formed in the side wall and is a light component flow outfall; and one opening at the axial end part is a supply inlet, and the other opening at the axial end part is a heavy component outfall. The devicehas the advantages that carbon isotope separation based on the molecular drainage principle can be remarkably realized, and the carbon isotope has high effective proportion in the separation medium.
Owner:TSINGHUA UNIV
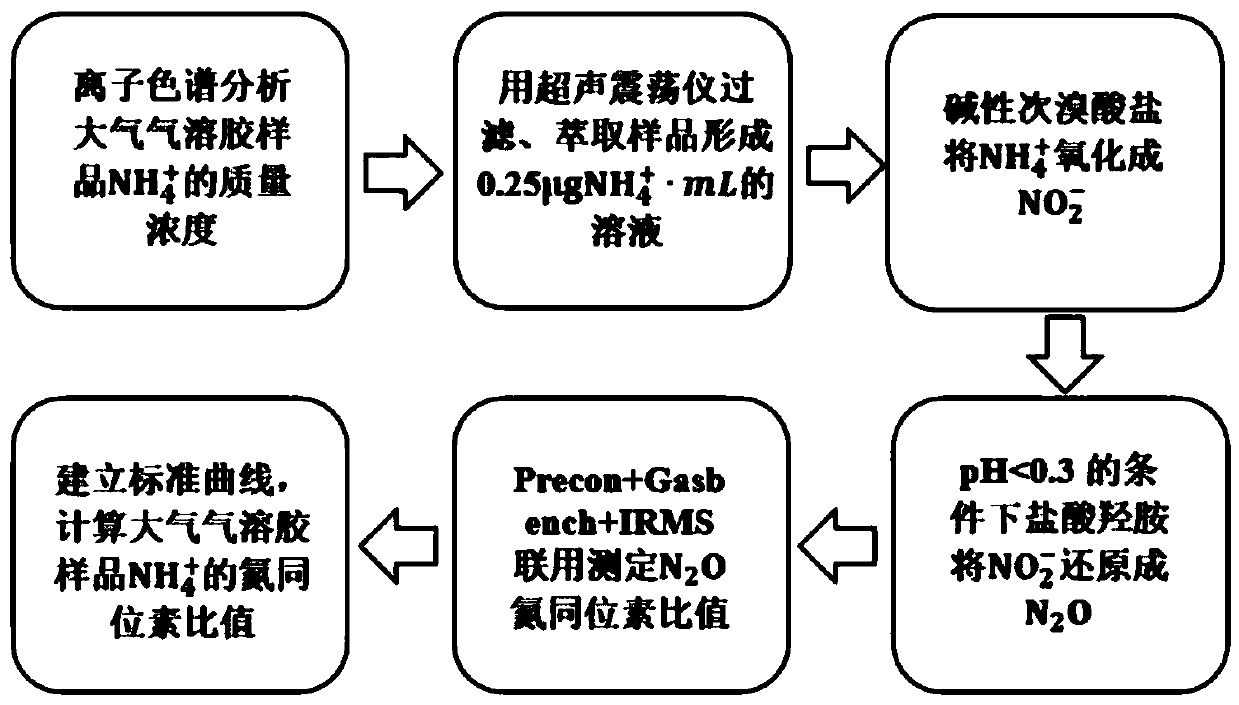
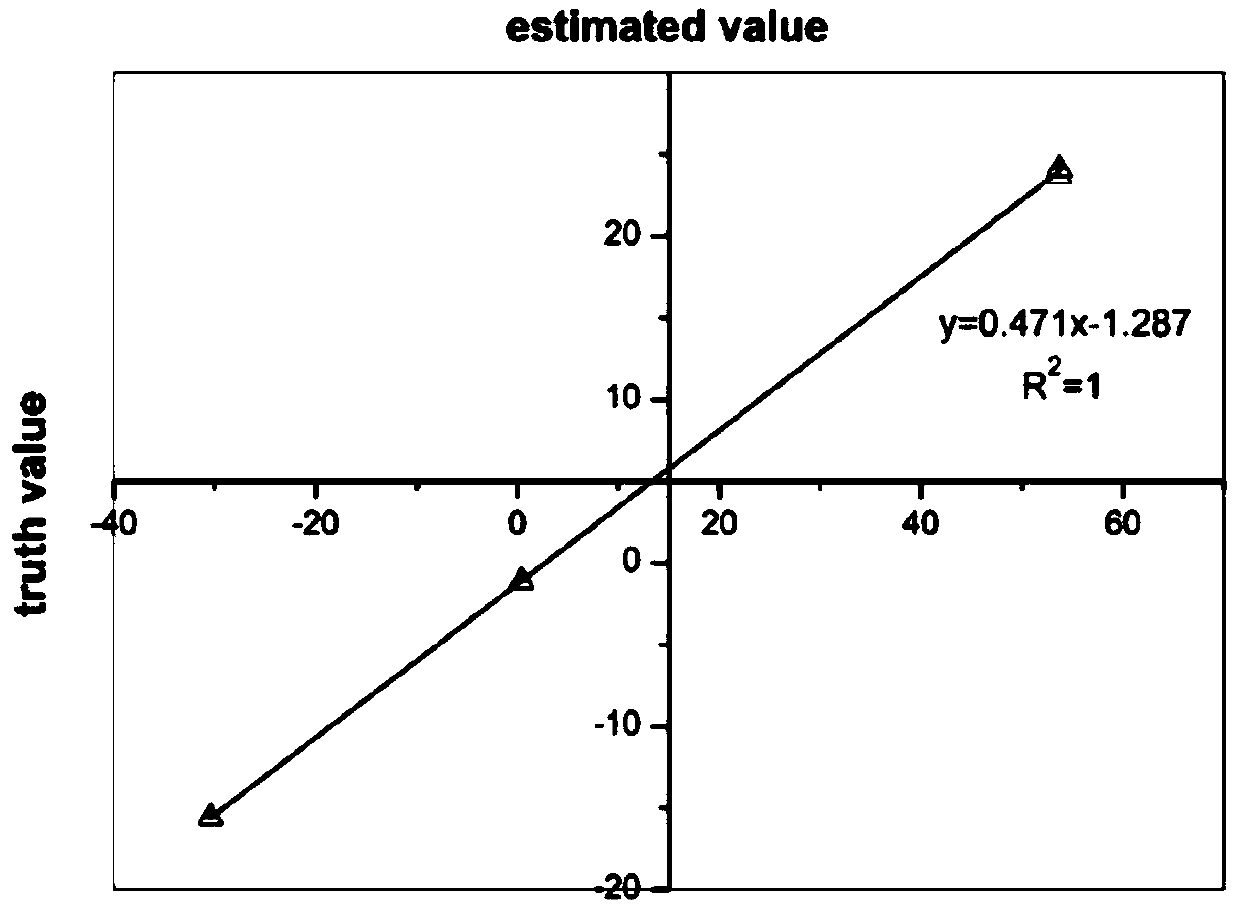
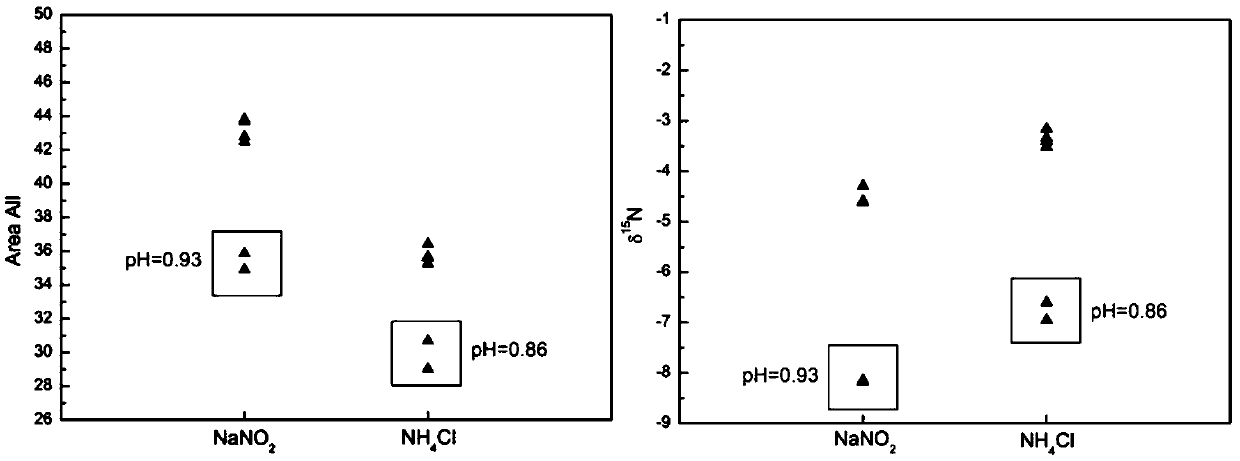
Method for measuring isotope ratio of ammonium nitrogen in atmospheric aerosol based on chemical conversion
InactiveCN108680697AImprove responseEasy to operateComponent separationIon chromatographyHydroxylamine Hydrochloride
The invention discloses a method for measuring isotope ratio of ammonium nitrogen in atmospheric aerosol based on chemical conversion, comprising the following steps: analyzing mass concentration of NH4<+> in an atmospheric aerosol sample by ion chromatography; filtering and extracting the sample by an ultrasonic oscillator to form 0.25 microgram NH4<+> / ml solution; oxidizing NH4<+> with alkalinehypobromite into NO2<->; reducing NO2<-> with hydroxylamine hydrochloride into N2O at pH of less than 0.3; measuring N2O nitrogen isotope ratio by Precon + GasBench II+IRMS combination; and establishing a standard curve and calculating nitrogen isotope ratio of NH4<+> in the atmospheric aerosol sample. The method of the invention has the following advantages: result accuracy is high, and accuracyafter repeating for 10 times reaches 0.005%.
Owner:NANJING UNIV OF INFORMATION SCI & TECH
Process and apparatus for isotope separation in low-gravity environment
InactiveUS6930304B2Avoid reactionReduce depthParticle separator tubesVapor condensationElectromagnetic radiationMicro gravity
A process and apparatus for separating element isotopes in a microgravity or low-gravity environment using electromagnetic radiation, e.g., sunlight, to heat and ionize a stream of raw materials, followed by electromagnetic separation, and collection of the desired isotopes in or on one or more collection surfaces or receptacles, such as a rotating surface. A cylindrical mirror can serve to collect and concentrate the electromagnetic radiation, permitting the stream of material to be heated and ionized while the path of the stream of material is oriented other than parallel to the direction of the radiation.
Owner:SCHUBERT PETER J
Method for separating boron isotopes by using metal-organic framework material as stationary phase of simulated moving bed
InactiveCN107020014AHigh separation factorLow costBoron/boridesIsotope separationSimulated moving bedMetal-organic framework
The invention discloses a method for separating boron isotopes by using a metal-organic framework material as a stationary phase of a simulated moving bed. The method comprises the steps of (1) respectively filtering aqueous solution of boric acid and aqueous solution of hydrochloric acid by a filter membrane, removing impurities and carrying out ultrasonic degassing; (2) dividing the simulated moving bed into I to IV regions, enabling each region formed by 3 to 7 chromatographic columns, using the metal-organic framework material as the stationary phase of the simulated moving bed, setting flow rates of the I to IV regions, port switching time and an operating temperature, continuously adding the aqueous solution of boric acid into the chromatographic columns of the simulated moving bed, carrying out elution by the aqueous solution of hydrochloric acid, and after the simulated moving bed reaches a balanced state, collecting aqueous solution of boric acid, which is enriched in an isotope 11B, at an extraction port of the simulated moving bed and collecting aqueous solution of boric acid, which is enriched in an isotope 10B, at an extraction raffinate port. The method is simple to operate and has higher separation efficiency; cost of concentrating the isotope 10B can be effectively reduced; the method has a high boron isotope separation factor, and the enrichment degree of the isotope 10B obtained by separation reaches 97.92% or more.
Owner:TIANJIN UNIV
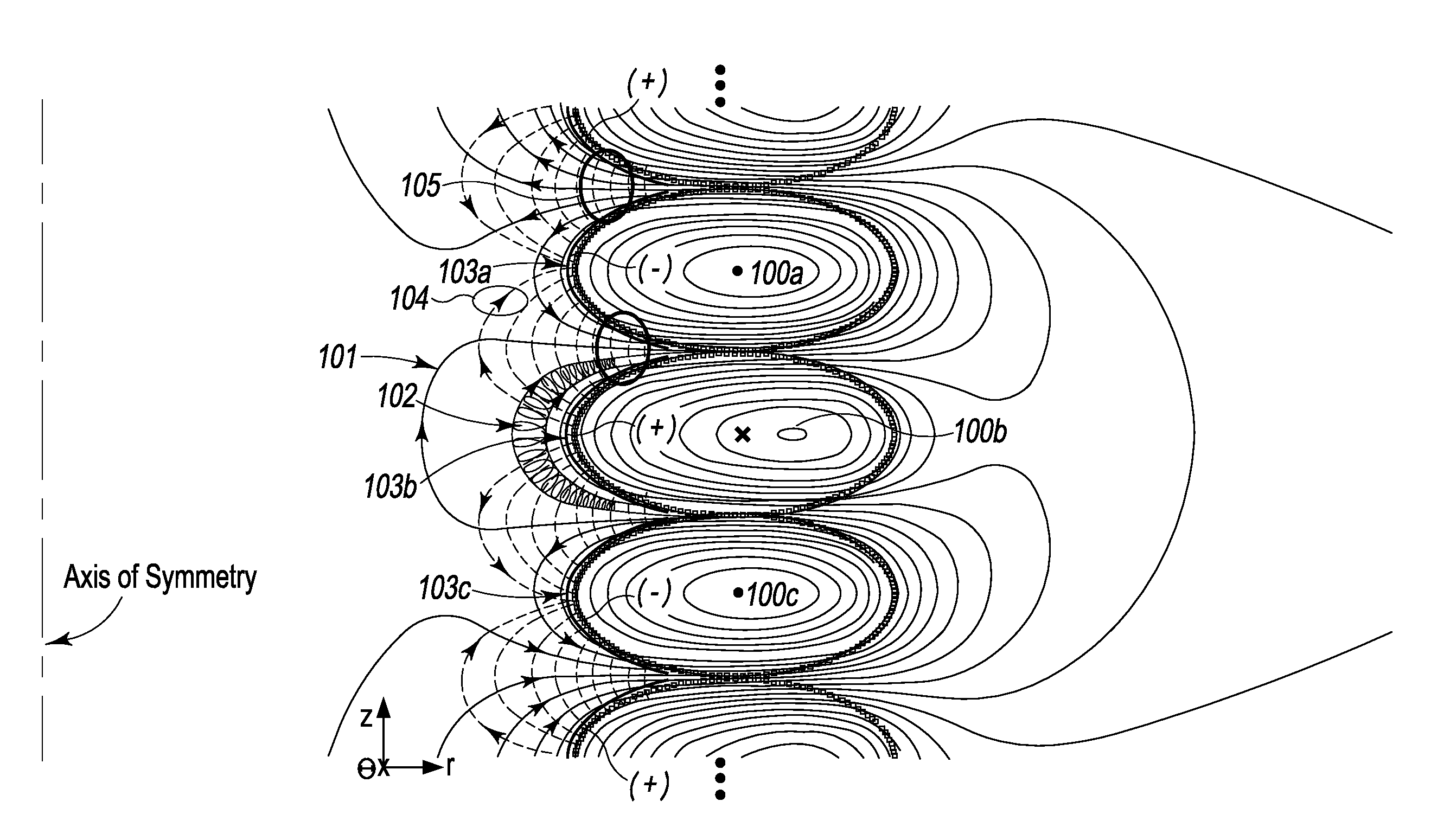
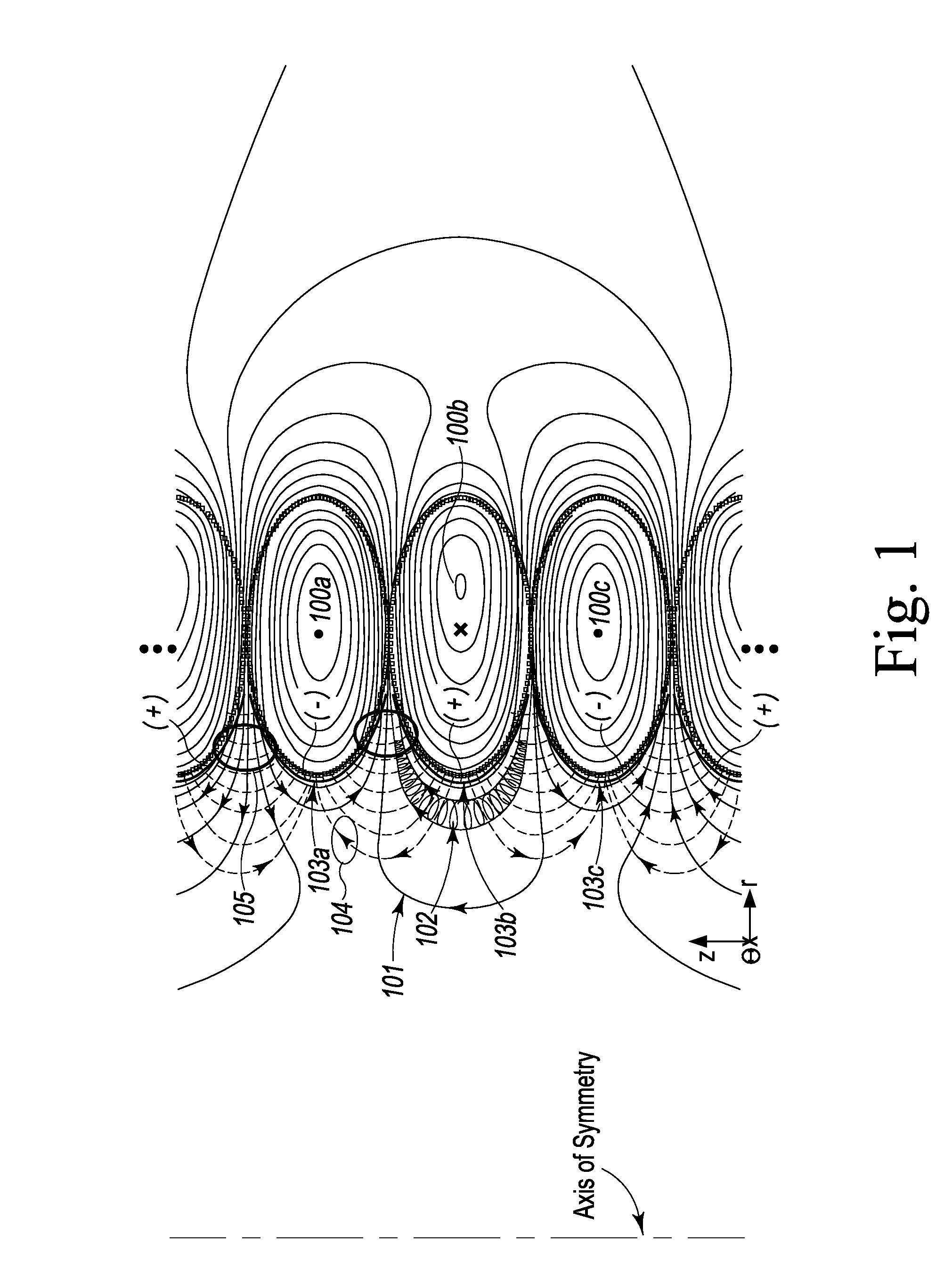
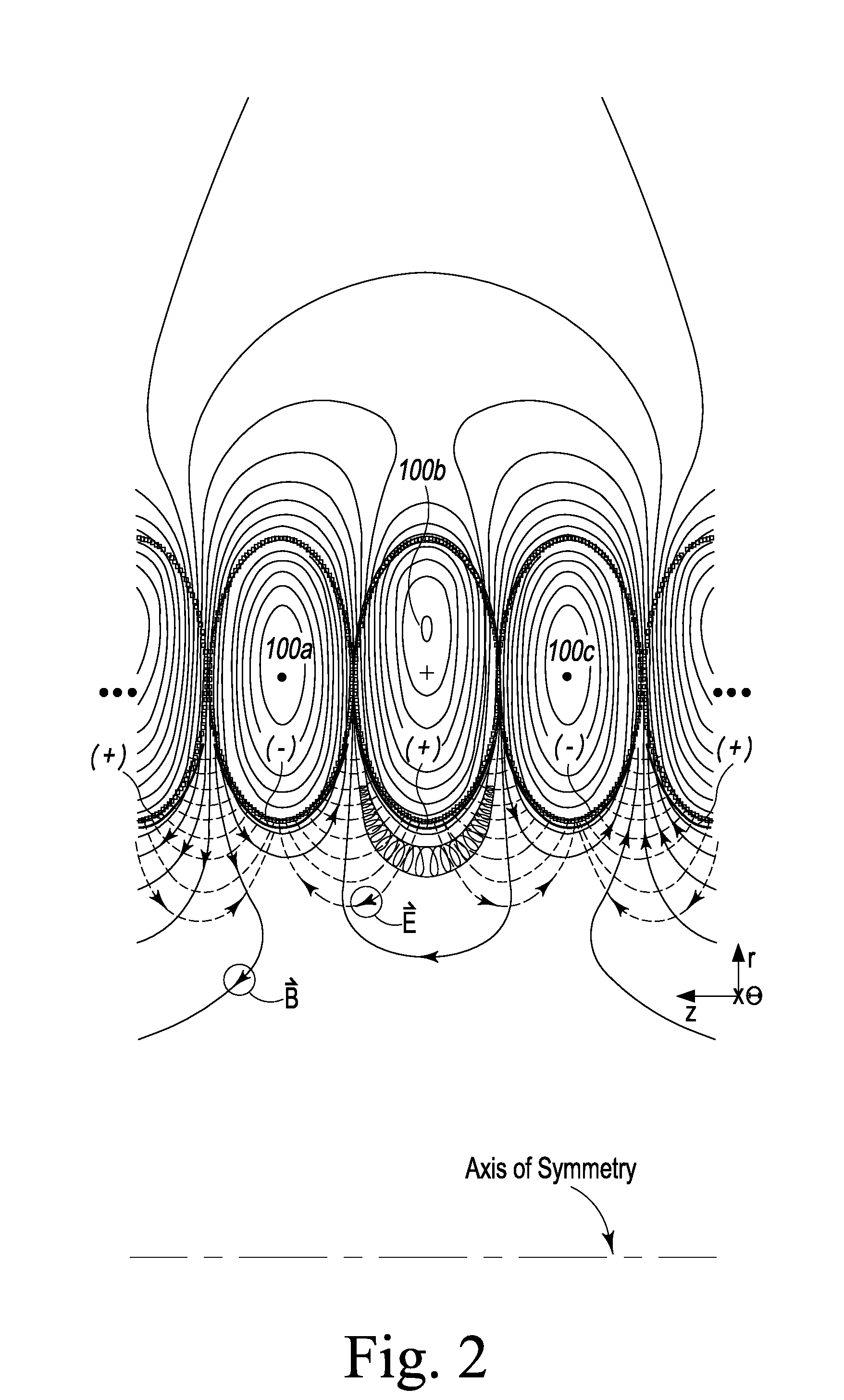
Plasma Confinement Device
A device and method for magnetic field confinement of plasma is formed by a cylindrically stacked column of direct current-carrying magnetic field coils with electrodes adjacently interior to each magnetic field coils so as to induce plasma rotation about an annular confinement region. Each field coil produces a magnetic field alternating in direction relative to adjacent coils and, so as to maintain consistent overall azimuthal direction of plasma rotation, electric field electrodes alternate accordingly in polarity along the axial length of the device. The device may be used for inducing nuclear fusion, for the ionic or isotopic separation of elements, creation of thrust by ejecting mass along the axial direction of the device, for creating a gravitational acceleration field, and for creating a state change beyond plasma.
Owner:PRATER DANIEL
Method for separating boron isotopes by using MOF-74(Zn) as simulated moving bed stationary phase
InactiveCN107413195AHigh separation factorLow costIsotope separationBoron oxyacidsSimulated moving bedMoving bed
The invention discloses a method for separating boron isotopes by using MOF-74(Zn) as a simulated moving bed stationary phase. The method comprises the following steps: firstly, filtering a boric acid aqueous solution and a hydrochloric acid aqueous solution, then removing impurities and degassing; secondly, a simulated moving bed comprises I to IV areas, wherein 3 to 7 chromatographic columns are arranged in each area; taking the MOF-74(Zn) as the simulated moving bed in stationary phase; setting the flow rates of the I to IV areas, port switching time and operating temperature; continuously adding the boric acid aqueous solution into the chromatographic columns of the simulated moving bed, and eluting with the hydrochloric acid aqueous solution; after the simulated moving bed reaches the equilibrium state, collecting the boric acid aqueous solution enriched with isotope 11B at an extraction opening of the simulated moving bed and collecting the boric acid aqueous solution enriched with isotope 10B at the extraction opening of the simulated moving bed. The method disclosed by the invention is a method for continuously separating the boron isotopes and has the advantages of simple operation and relatively-high separation efficiency; in addition, the cost of concentrated isotope 10B can be effectively reduced and relatively-high boron isotope separation factors are obtained.
Owner:TIANJIN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com