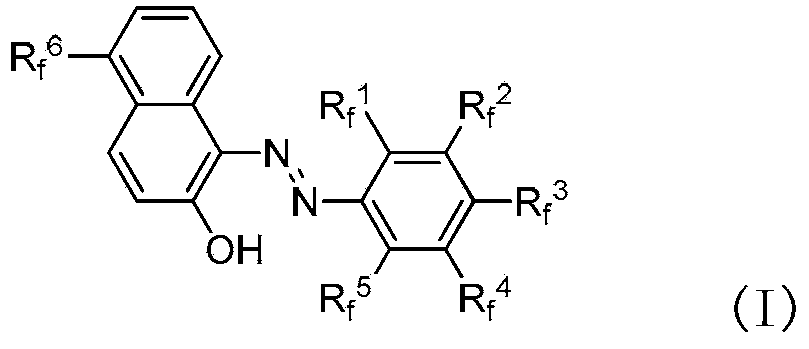
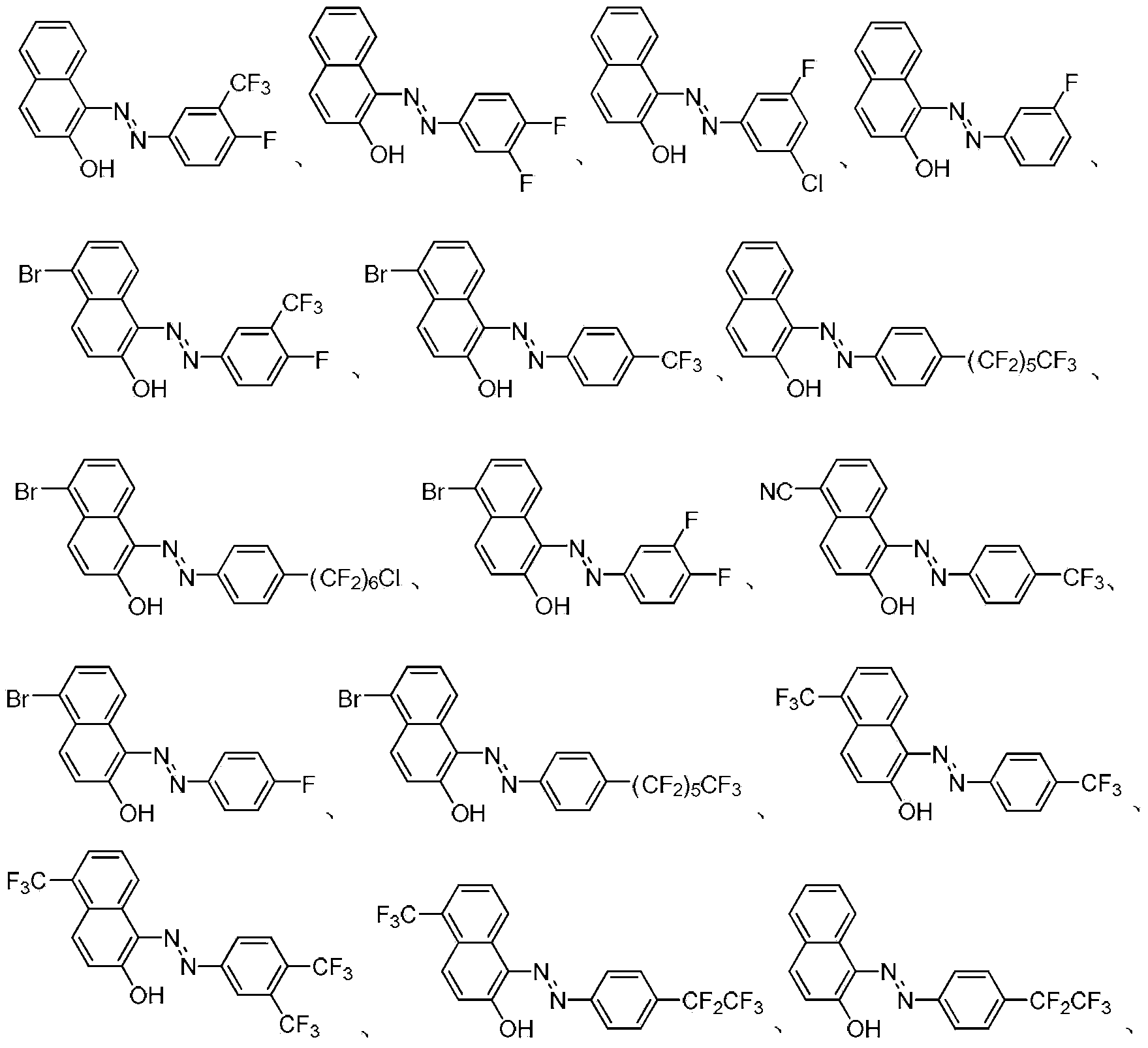
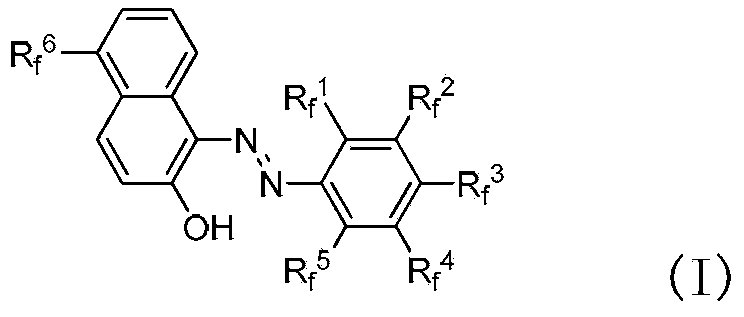
Fluorine-containing extractant and application thereof
An extraction agent and extraction technology, applied in the chemical industry, can solve problems such as experiments without multi-stage enrichment and separation, low extraction rate, operators and environmental hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151] Embodiment 1 single-stage extraction separation test
[0152] extraction:
[0153] Extract the organic phase:
[0154] Extracting agent: 0.4mol / L; co-extractant: 0.2mol / L quaternary ammonium salt N263; diluent: chloroform.
[0155] Water phase: 0.2mol / L LiCl; 0.5mol / L NaOH
[0156] Comparison: 1:1 (volume ratio of organic phase to aqueous phase)
[0157] Extract the organic phase and aqueous phase into a separatory funnel and shake for 5 minutes, stand still for 5 minutes to separate layers, collect the two phases as the extracted organic phase and aqueous phase respectively; or use a high-efficiency centrifugal extractor to continuously extract at a flow ratio of 1:1 Feeding and discharging, the two phases obtained from the outlet are respectively used as the extracted organic phase and the aqueous phase.
[0158] in the organic phase after extraction 7 Li / 6 Li=14.350, in the water phase after extraction 7 Li / 6 Li=14.096, that is, the single-stage separatio...
Embodiment 2
[0162] Embodiment 2 single-stage extraction separation test
[0163] extraction:
[0164] Extraction method is the same as embodiment 1, and difference is to adopt following condition:
[0165] Extract the organic phase:
[0166] Extracting agent: 0.8mol / L; co-extractant: 0.4mol / L methyl tri-n-octyl ammonium bis(trifluoromethanesulfonyl)imide; diluent: chlorobenzene.
[0167] Water phase: 0.25mol / L LiOH; 0.2mol / L NaOH
[0168] Compared to: 1:1
[0169] The extraction distribution ratio D=3.4 was measured, that is, the primary extraction rate was 77%, and the single-stage separation coefficient α value was 1.016.
[0170] Stripping:
[0171] Back extraction method is the same as embodiment 1, and difference is to adopt stripping agent: 2mol / L H 2 SO 4 ; Three stripping rates were measured greater than 99%.
Embodiment 3
[0172] Embodiment 3 single-stage extraction separation test
[0173] extraction:
[0174] Extraction method is the same as embodiment 1, and difference is to adopt following condition:
[0175] Extract the organic phase:
[0176] Extracting agent: 0.12mol / L; co-extractant: 0.08mol / L quaternary ammonium salt N263; diluent: chloroform.
[0177] Water phase: 0.06mol / L LiOH; 0.6mol / L NaOH
[0178] Compared to: 1:1
[0179] The extraction distribution ratio D=1.6 was measured, that is, the primary extraction rate was 62%, and the single-stage separation coefficient α value was 1.019.
[0180] Stripping:
[0181] Back extraction method is the same as embodiment 1, and difference is to adopt stripping agent: 2mol / L H 2 SO 4 ; Three stripping rates were measured greater than 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com