Non-woven base composite membrane for lithium isotope separation, and preparation method thereof, as well as lithium isotope separation method by using membrane chromatography
A lithium isotope and composite membrane technology, which is applied in the field of membrane materials and lithium isotope separation, can solve the problems of difficult elution of lithium isotopes and low separation efficiency of lithium isotopes, and achieves easy continuous production, realizes continuous production, membrane stack pressure reduced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
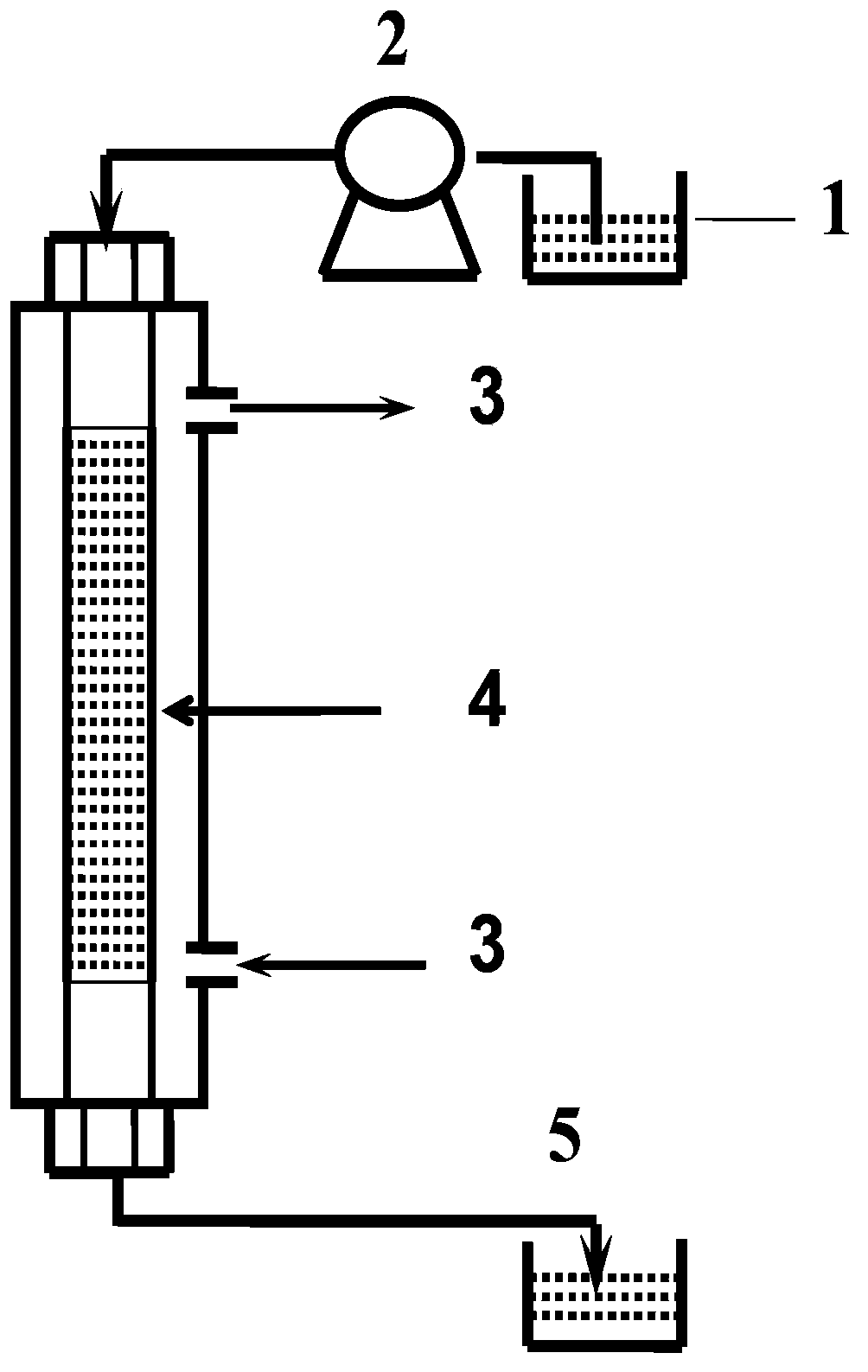
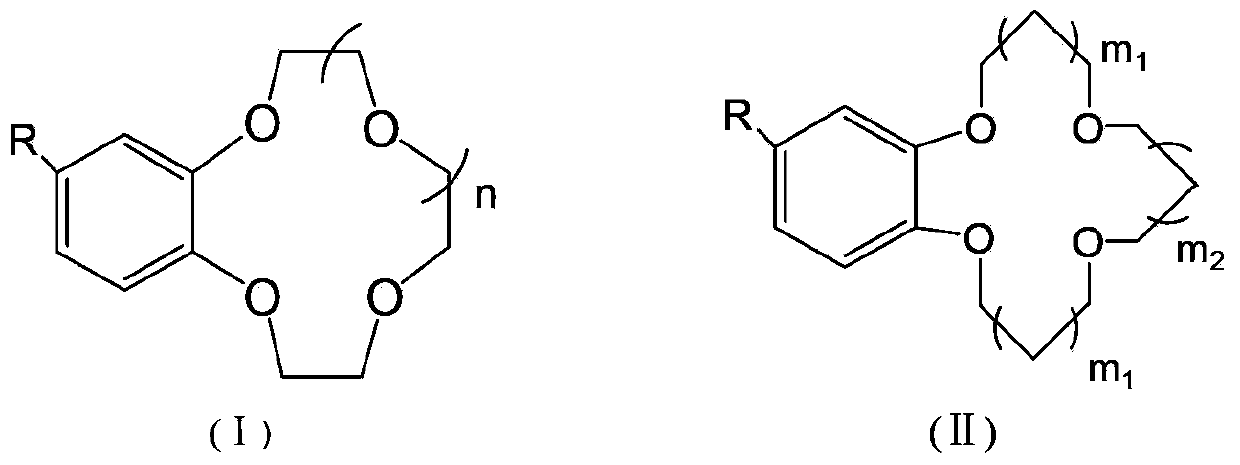
[0057] Using 4-aminobenzo15crown5-grafted polysulfone polymer material (capacity of crown ether is 0.1mmol / g) to prepare a non-woven matrix composite membrane for lithium isotope separation, the preparation method includes the following steps: first, use alkali Catalytic hydrolysis method is carried out modification to polyester nonwoven fabric (modification treatment step: the ethanol solution of the NaOH of 7g / L, the aqueous solution of 0.5g / L fixation accelerator, described fixation accelerator is hexadecyl Trimethylammonium bromide; put the non-woven fabric into the container, add the ethanol solution of NaOH and the aqueous solution of the fixation accelerator at a ratio of 70:1 by volume, and then heat it in a constant temperature water bath at 40°C for 2 hours, and take it out And soak in ethanol for 2 hours to remove the fiber surface solution; then place the sample in an electric heating constant temperature blast drying oven to dry), cut out a certain area of the mo...
Embodiment 2
[0063] Composite membranes were prepared by impregnation as described in Example 1, except that the 4-aminobenzo 15 crown 5 grafted polysulfone polymers were replaced by 4-hydroxybenzo 15 crown 5 grafted polysulfone polymers (crown Ether immobilization capacity is 2.0mmol / g), 4-aminobenzo 12 crown 4 graft polysulfone polymer (crown ether immobilization capacity is 1.0mmol / g), 4-hydroxybenzo 14 crown 4 graft polysulfone Polymer (capacity of crown ether is 0.8mmol / g), 4-aminobenzo18 crown 6 grafted polysulfone polymer (capacity of crown ether is 0.6mmol / g), 4-formylbenzo13crown 4-grafted polyvinyl alcohol (capacity of crown ether is 3.0mmol / g), 4-formylbenzo15crown 5-grafted polyvinyl alcohol (capacity of crown ether is 1.1mmol / g), 4-formyl Benzo-12-crown 4-grafted polyvinyl alcohol (capacity of crown ether is 0.7mmol / g), 4-formylbenzo14crown-4 grafted polyvinyl alcohol (capacity of crown ether is 2.0mmol / g), The above-mentioned graft polymers are respectively made of dimethyls...
Embodiment 3
[0065] To prepare a nonwoven-based composite membrane for lithium isotope separation, the preparation method includes the following steps: firstly, the nonwoven fabric is modified by alkali-catalyzed hydrolysis (the modification treatment steps are the same as in Example 1), and the modified nonwoven fabric is cut into Take out a certain area, soak it in absolute ethanol for 24 hours to remove impurities on the surface of the membrane, and take it out to dry. 4-Aminobenzo21crown 7 grafted polysulfone polymer (capacity of crown ether is 0.2mmol / g), 4-aminobenzo15crown5 grafted polyetheretherketone polymer (capacity of crown ether is 1.2mmol / g), 4-hydroxybenzo15crown5 grafted polyetheretherketone polymer (crown ether solid loading is 0.7mmol / g), 4-aminobenzo15crown5 grafted polyethersulfone polymer (crown ether solid loading is 1.2mmol / g), 4-carboxybenzo 18 crown 6 grafted polyethylene-vinyl alcohol (crown ether solid loading is 2.6mmol / g), 4-carboxybenzo 21 crown 7 graft Branc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com