Purification method of chlorine trifluoride
A technology of chlorine trifluoride and purification method, which is applied in the direction of halogen compounds, etc., can solve the problems of material selection and use of purification equipment, and achieve the effects of reducing the content of impurities, high purification efficiency and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
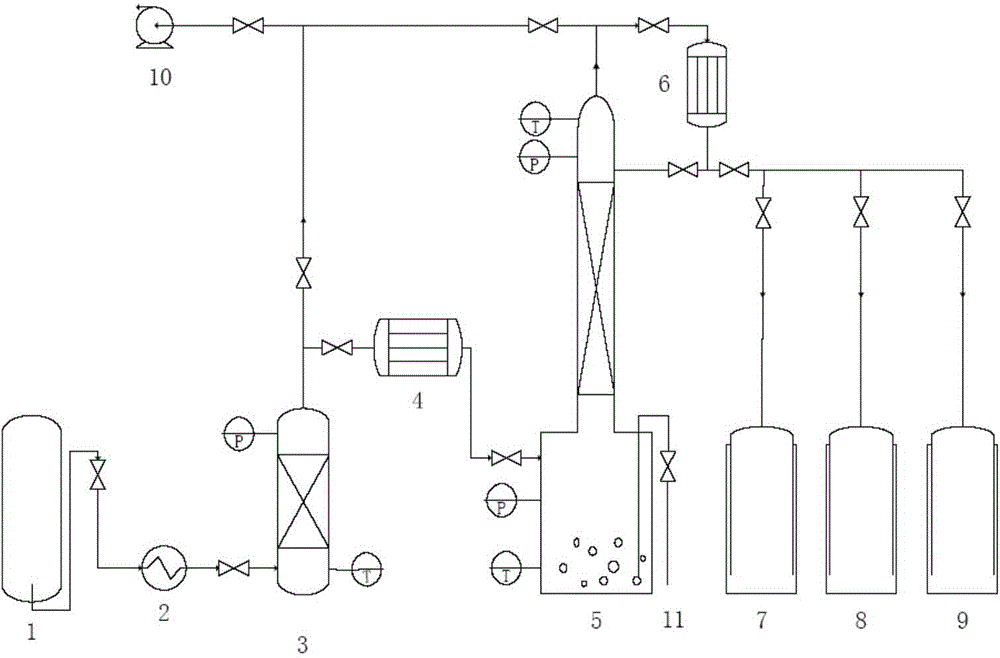
[0034] (1) The entire purification system is deoiled, dried and vacuumized, and replaced with high-purity nitrogen for 5 times to ensure that there are no impurities other than nitrogen in the system. Fill the adsorption tower with spherical sodium fluoride particles with a diameter of 1mm and a porosity of 10%, with a filling height of 450mm, and inject high-purity nitrogen to activate sodium fluoride at 500°C for 10h.
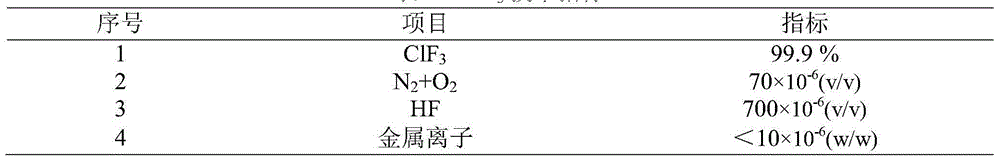
[0035] (2) vaporize the chlorine trifluoride solution (the content of chlorine trifluoride is 91%) in the crude product storage tank by a vaporizer, in the vaporizer, water is used as a heat-conducting agent, and the water temperature is 16°C, and the vaporized chlorine trifluoride (at a temperature of 15° C.) into an adsorption tower filled with fillers at a flow rate of 0.4 L / min, and stay in the adsorption tower for 0.1 min at 20° C. and 0.01 MPa to obtain a grade 1 crude product of chlorine trifluoride;
[0036] (3) pass the first-grade crude product of c...
Embodiment 2
[0041] (1) The entire purification system is deoiled, dried and vacuumized, and replaced with high-purity nitrogen for 5 times to ensure that there are no impurities other than nitrogen in the system. Fill the adsorption tower with spherical potassium fluoride particles with a diameter of 10 mm and a porosity of 30%, with a filling height of 700 mm, and inject high-purity nitrogen to activate potassium fluoride at 300 °C for 6 hours.
[0042] (2) the chlorine trifluoride solution (the content of chlorine trifluoride is 95%) in the crude product storage tank is vaporized by a vaporizer, and water is used as a heat conducting agent in the vaporizer, and the water temperature is 60 ℃, and the vaporized chlorine trifluoride (at a temperature of 40° C.) into an adsorption tower filled with fillers at a flow rate of 0.9 L / min, and stay in the adsorption tower for 5 minutes at 40° C. and 0.27 MPa to obtain a first-grade crude product of chlorine trifluoride;
[0043] (3) pass the fir...
Embodiment 3
[0048] (1) The entire purification system is deoiled, dried and vacuumized, and replaced with high-purity nitrogen for 5 times to ensure that there are no impurities other than nitrogen in the system. Fill the adsorption tower with spherical potassium fluoride particles with a diameter of 12mm and a porosity of 80% to a filling height of 700mm, and pass high-purity nitrogen to activate potassium fluoride at 400°C for 8 hours.
[0049] (2) Vaporize the chlorine trifluoride solution (the content of chlorine trifluoride is 95%) in the crude product storage tank by a vaporizer, in the vaporizer, heat transfer oil is used as a heat transfer agent, and the temperature of the heat transfer oil is 90°C, and the vaporized trifluoride Chlorine fluoride (at a temperature of 50° C.) is passed into an adsorption tower filled with packing at a flow rate of 1.8 L / min, and is retained in the adsorption tower at 80° C. and 1 MPa for 5 minutes to obtain a first-grade crude product of chlorine tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com