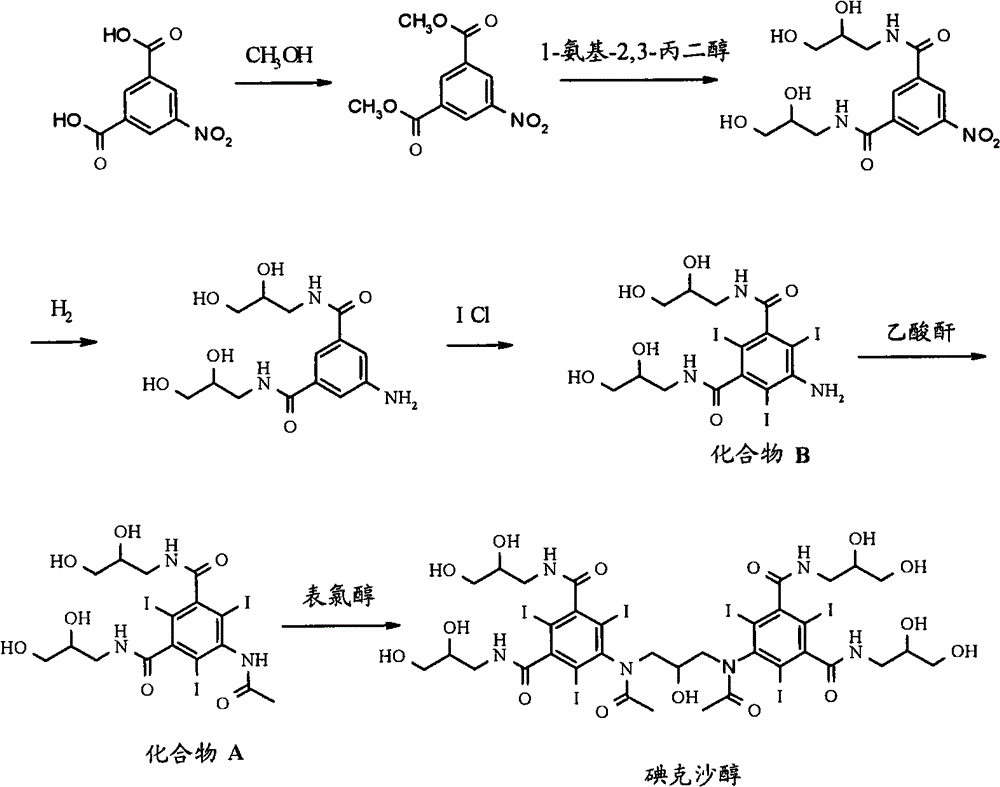
Synthesis of iodixanol in water
A technology of iodine and triiodophenyl is applied in the field of synthesizing iodixanol in water, and can solve the problems of low concentration and high impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
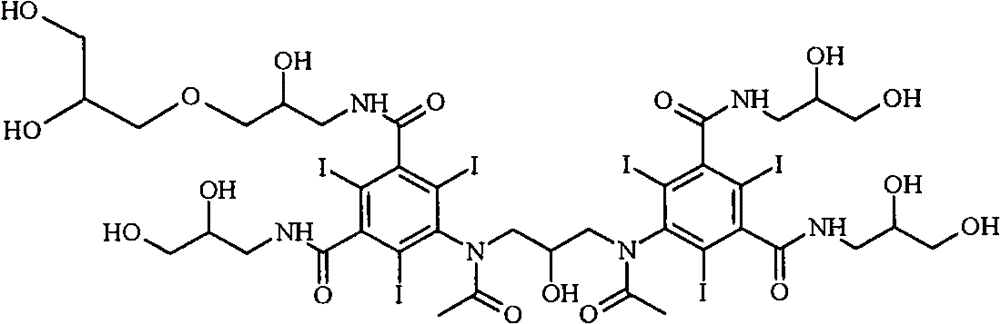
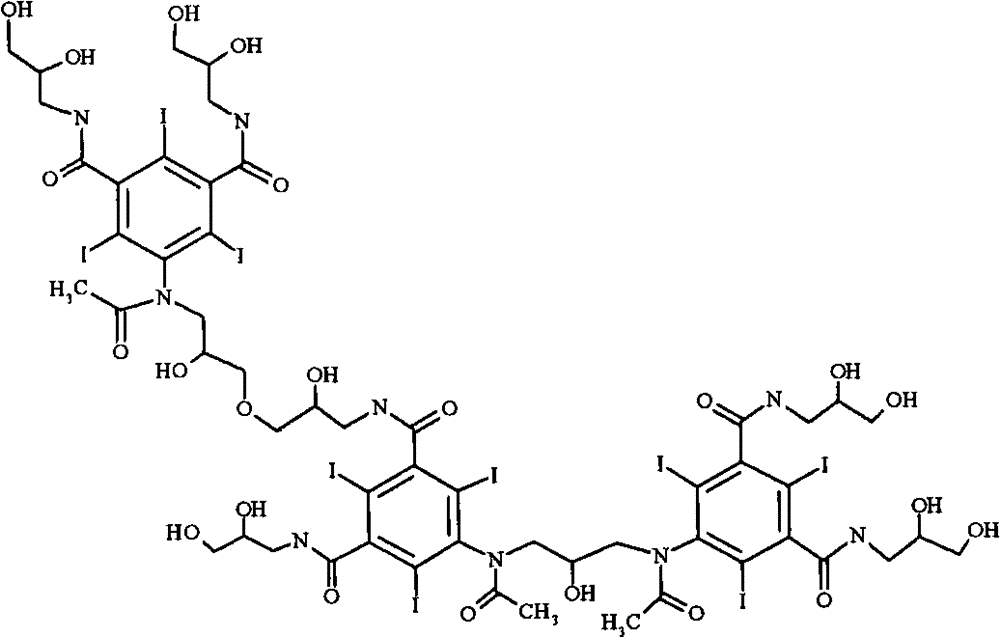
[0021] The most important impurities in reactions with regard to work-up importance are the so-called backpeaks. The term refers to retention times in reverse phase HPLC where the latter peak has a slightly longer retention time than iodixanol itself. Most of the back peaks are trimers or O-alkylated dimers. Two examples are as follows:
[0022]
[0023] primary (primary) O-alkyl iodixanol
[0024]
[0025] Iodixanol Primary O-Alkyl Trimer
[0026] Other important by-products are eg iohexol and N-acetylcyclized iodixanol, the structures of which are shown below. Iohexol is relatively easy to remove during the subsequent crystallization of iodixanol, even when it is present at a few weight percent.
[0027]
[0028] N-acetyl cyclized iodixanol
[0029]
[0030] iohexol
[0031] Typical selectivities required to enable economically viable work-up and obtain the desired product quality are 50-60% conversion of Compound A to iodixanol with no more than 2% of the ...
Embodiment 1
[0049]Dissolve sodium hydroxide pellets (1.19eq.) in water (250ml), add 5-acetamido-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo - Isophthalamide (compound A) (50 g). The pH was adjusted from 12.7 to 12.2 with 2M hydrochloric acid, the mixture was cooled to 20°C, and epichlorohydrin (0.27eq) was added in 3 portions over 70 minutes. HPLC analysis after 48 hours showed the following composition: 43.5% iodixanol, 0.8% back peak and 5.1% iohexol.
Embodiment 2
[0051] Dissolve sodium hydroxide pellets (1.19eq.) in water (150ml), add 5-acetamido-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo - Isophthalamide (compound A) (50 g). The pH was adjusted from 12.7 to 12.2 with 2M hydrochloric acid, the mixture was cooled to 20°C, and epichlorohydrin (0.27eq) was added in 3 portions over 70 minutes. HPLC analysis after 24 hours showed the following composition: 45.6% iodixanol, 1.4% back peak and 4.6% iohexol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com