Stabilizing aqueous solution of iodine chloride by adding sodium chloride
A technology of iodine chloride and sodium chloride, applied in the direction of interhalogen compounds, etc., can solve problems such as reducing the use efficiency and reducing the concentration of iodine chloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
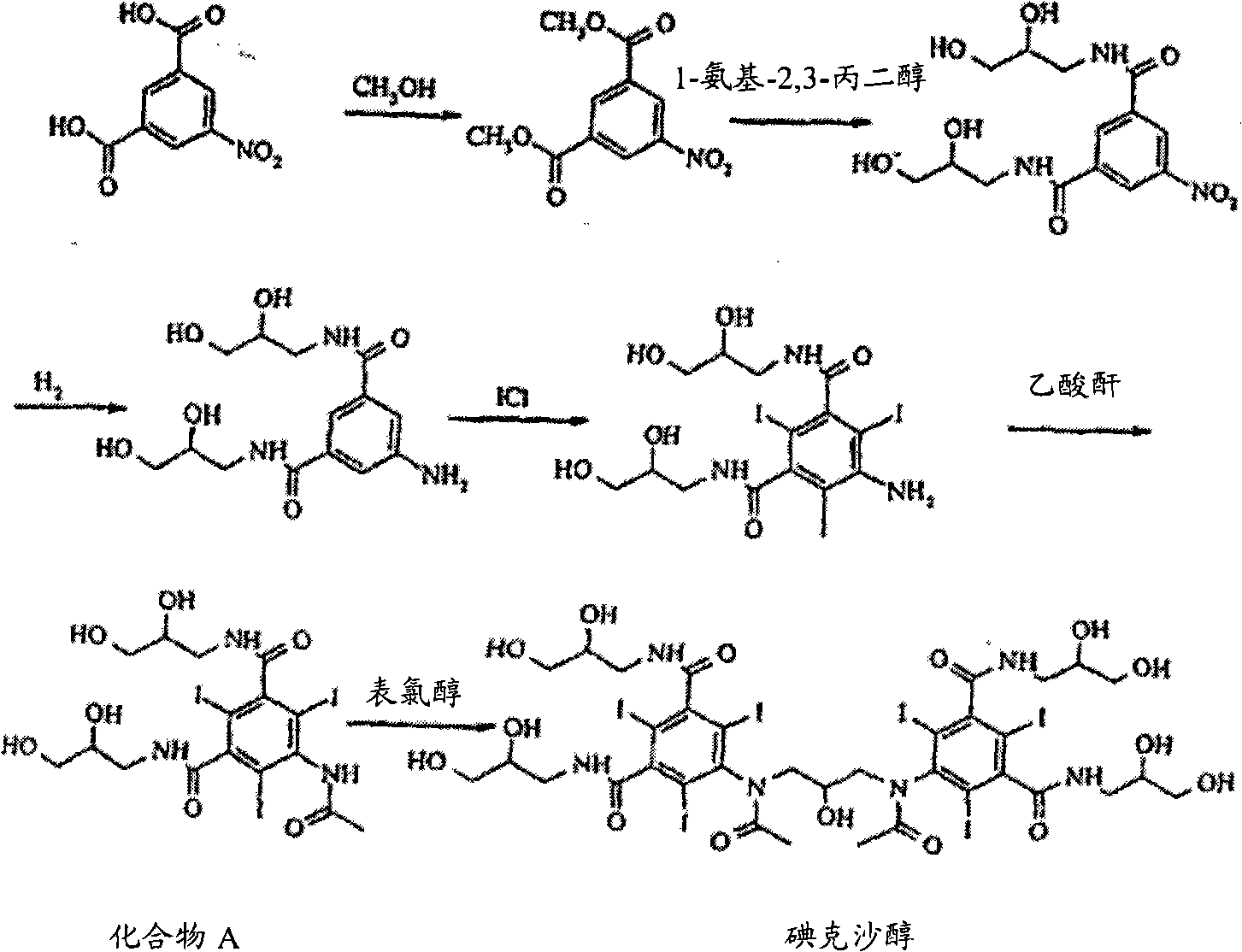
Image
Examples
Embodiment 1
[0019] Iodine (7.61 kg, 30 mol), hydrochloric acid (37%, 6.91 kg, 70 mol), water (0.62 kg, 35 mol) and sodium chloride (1.46 kg, 25 mol) were mixed in a reactor at 25°C. A solution of sodium chlorate (1.07 kg, 10 mol) in water (1.20 kg, 67 mol) was prepared with heating and added carefully to the reactor over a period of 2 hours with vigorous stirring. The reaction was continued at 27-30°C for 17 hours with moderate stirring. Free iodine was confirmed by extracting the sample with chloroform in the presence of hydrochloric acid (a pink color was obtained). The pH was measured to be 1.06. An aqueous iodine chloride solution (18.80 kg) having a concentration of 51.6 w / w% (relative to the reaction solution) was obtained, corresponding to a yield of 99.6%.
Embodiment 2
[0021] Iodine (2700kg, 10.6kmol), hydrochloric acid (35%, 2285kg, 21.9kmol), water (250kg, 13.3kmol) and sodium chloride (475kg, 8.1kmol) were mixed in a reactor at 20°C. Under vigorous stirring, an aqueous solution of sodium chlorate (43 w / w%, 864 kg, 2.6 mol) was carefully added to the reactor over 3-4 hours. The reaction was continued at 50°C for at least 10 hours with moderate agitation. Free iodine was confirmed by extracting the sample with chloroform in the presence of hydrochloric acid (organic phase was pink). The resulting pH is below 0.5. An aqueous solution of iodine chloride (approximately 3970 kg) was obtained at a concentration of 50 w / w% (relative to the reaction solution), corresponding to a yield of almost 100% relative to iodine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com