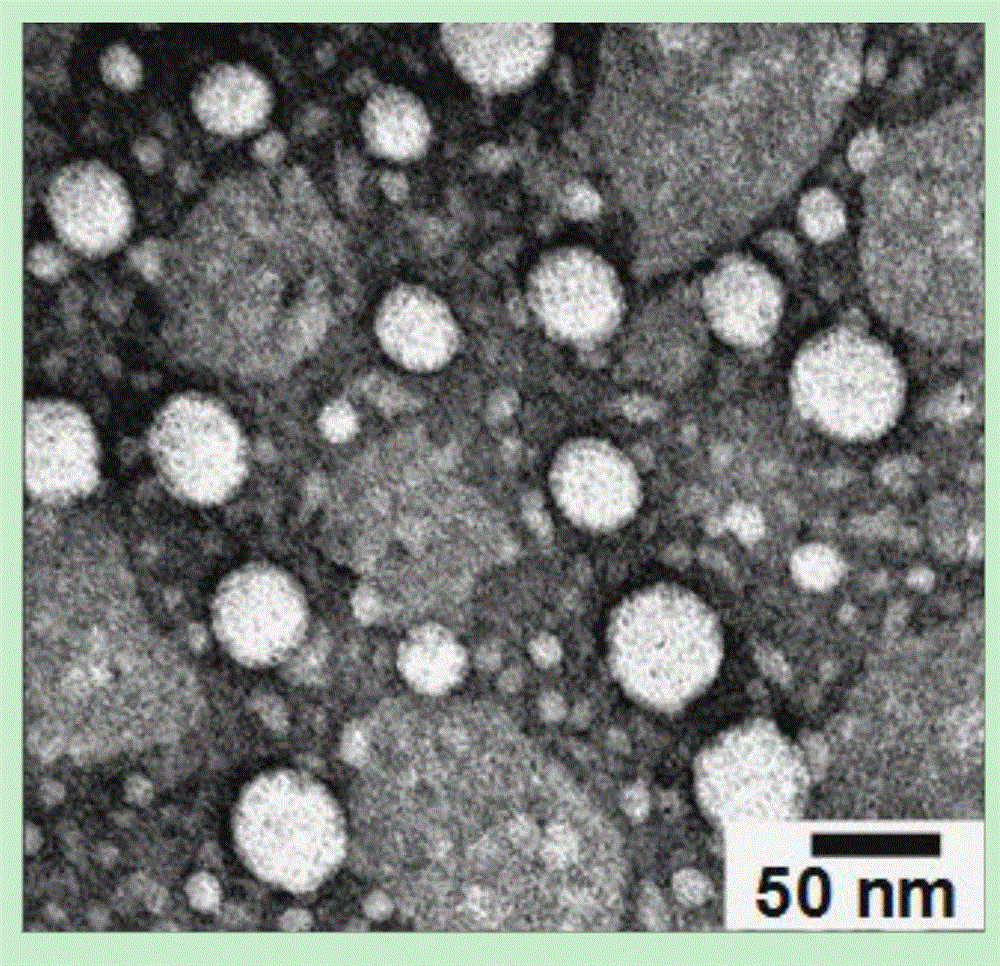
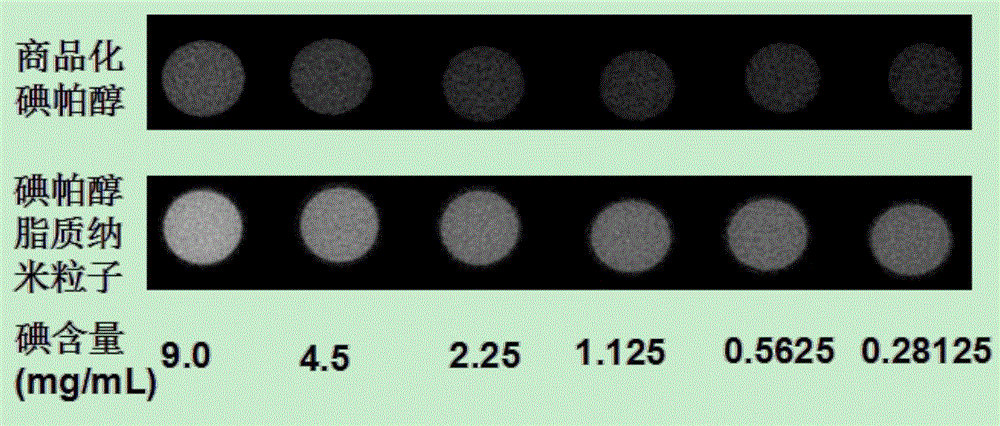
Contrast medium based on iopamidol lipid derivative as well as preparation method and application of contrast medium
A lipid derivative, iopamidol technology, applied in the field of biomedical materials, can solve the problems of easy and rapid extravasation and removal, large size, and removal, and achieve the effect of cheap raw materials, strong operability, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Dissolve 20mmol of compound 1 in 70mL of toluene, then add 1.2mmol of compound 2 and 0.1mmol of concentrated sulfuric acid in sequence, stir at 105°C for 48 hours, and spin the solvent to dry. The obtained crude product is separated and purified by column chromatography to obtain compound 3 , yield 53.5%.C 21 h 40 o 4 1 H NMR (CDCl 3 ,400MHz)δ:0.88(t,3H,CH 3 ),1.26-1.31(m,28H,CH 2 ), 1.49(t,3H,CH 3 ), 1.64(t,2H,CH 2 ),2.35(t,2H,CH 2 ), 5.01-5.03 (m, 1H, CH). Theoretical value of mass spectrum MS: 356.29, experimental value [M] + :357.3.
Embodiment 2
[0030] Under the protection of nitrogen, 1 mmol of compound 3 was dissolved in 50 mL of anhydrous toluene, then slowly added dropwise to 5 mmol of thionyl chloride, stirred at 80 ° C for 36 hours, and the solvent was spin-dried, and the obtained crude product was mixed with petroleum ether / acetone Compound 4 was purified by solvent recrystallization with a yield of 65.2%.C 21 h 39 ClO 3 1 H NMR (CDCl 3 ,400MHz)δ:0.88(t,3H,CH 3 ),1.26-1.32(m,28H,CH 2 ), 1.47(t,3H,CH 3 ),1.62-1.65(m,2H,CH 2 ),2.35(t,2H,CH 2 ),5.00-5.04(m,1H,CH).MS theoretical value: 374.99, experimental value [M] + :375.8.
Embodiment 3
[0032] Under the protection of nitrogen, 1 mmol of compound 4 and 1.3 mmol of compound 5 were mixed, dissolved in 40 mL of DMA, stirred at 50°C for 60 hours, and evaporated to dryness under reduced pressure. The obtained crude product was separated and purified by column chromatography to obtain compound 6, Yield 75%.C 29 h 40 Cl 2 I 3 NO 5 1 H NMR (CDCl 3 ,400MHz)δ:0.88(t,3H,CH 3 ),1.26-1.32(m,28H,CH 2 ),1.53(t,3H,CH 3 ),1.63-1.65(m,2H,CH 2),2.36(t,2H,CH 2 ), 5.07-5.10 (m, 1H, CH). MS theoretical value: 934.25, experimental value [M] + :935.1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com