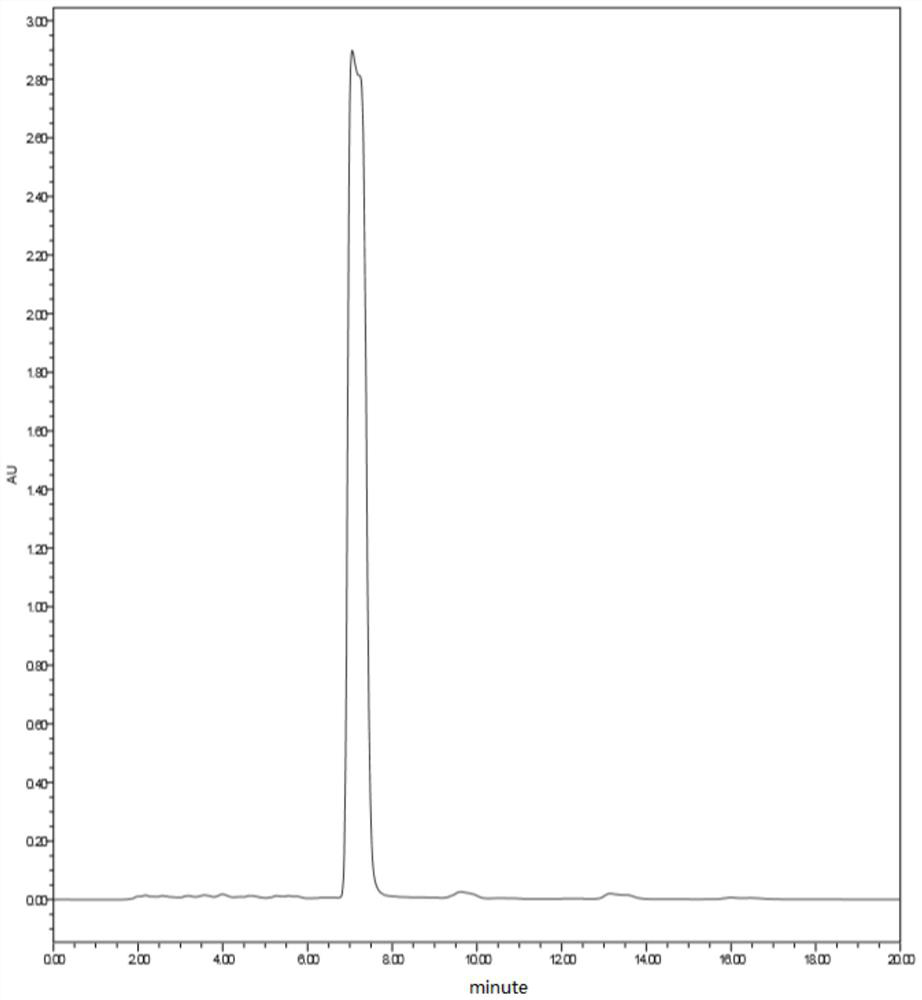
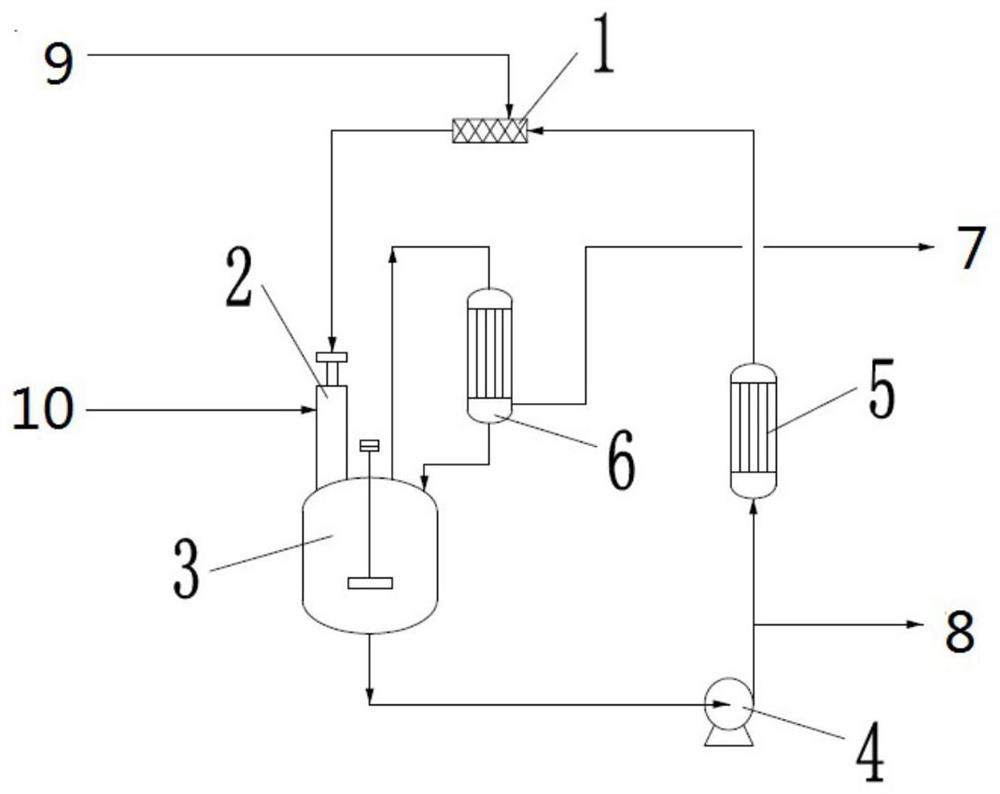
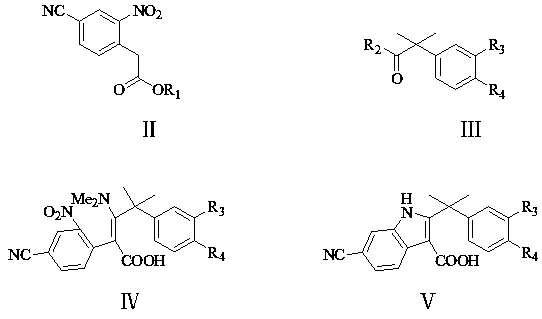
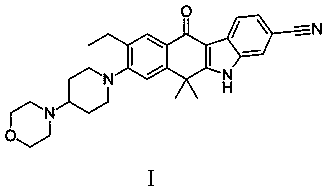
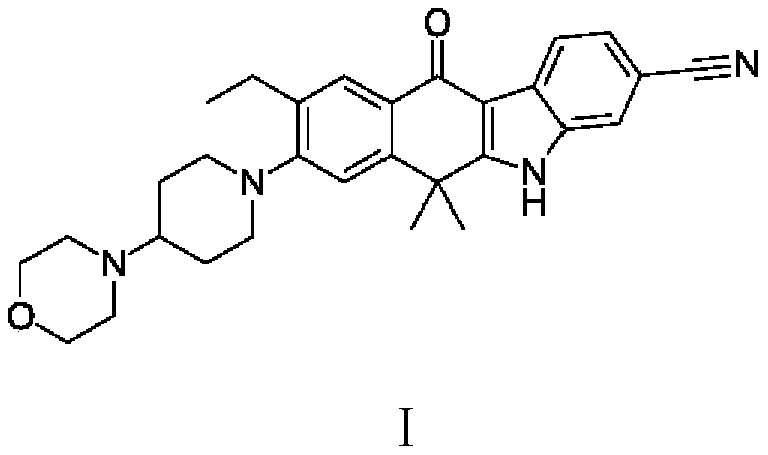
Patents
Literature
53 results about "Propanoyl chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Propanoyl chloride is an acyl chloride with a three-carbon chain. It shows the characteristic reactions of acyl chlorides. It is a colorless, corrosive, volatile liquid.
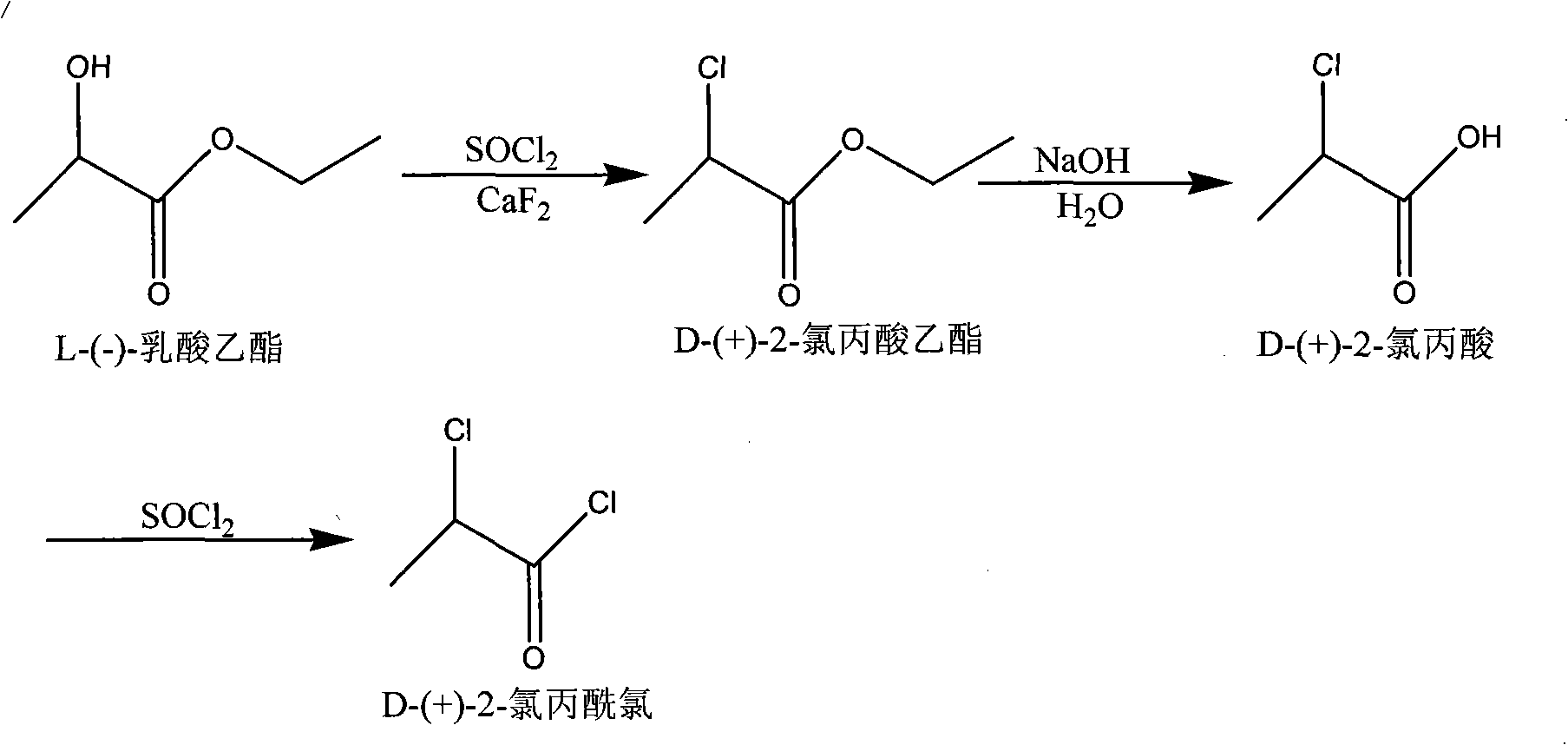
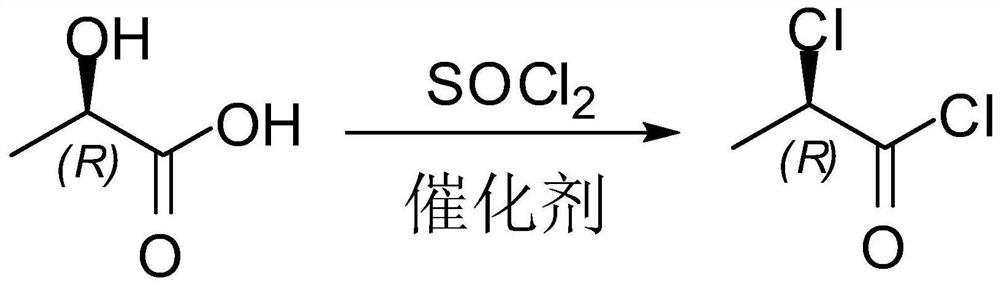
Synthetic method of D-(+)-2-chloro-propanoyl chloride
InactiveCN101284772ASettlement yieldSolve productivityOrganic compound preparationCarboxylic compound preparationPropanoyl chloride2-Chloropropionic acid
The invention relates to a method for synthesizing D-(+)-2-chloro-Propanoyl chloride, which is used for solving the problems that the product recovery rate of the prior synthetic method is low and the synthetic method is not suitable for industrial production. The method adopts the technical proposal that L-ethyl lactate and thionyl chloride react under the action of calcium fluoride catalyst to generate D-(+)-2-chloropropionic acid ethyl ester, D-(+)-2-chloropropionic acid ethyl ester is hydrolyzed under the action of caustic soda dissolving to generate D-(+)-2-chloropropionic acid, and D-(+)-2-chloropropionic acid reacts with the thionyl chloride to generate D-(+)-2-chloro-Propanoyl chloride. The method has the advantages that the method is novel, the process is simple, the product recovery rate is high, the cost of the catalyst is low, the catalyst is easy to obtain, the environment can not be affected, and the method is suitable for industrial production.
Owner:HEBEI HUACHEN PHARMA +1
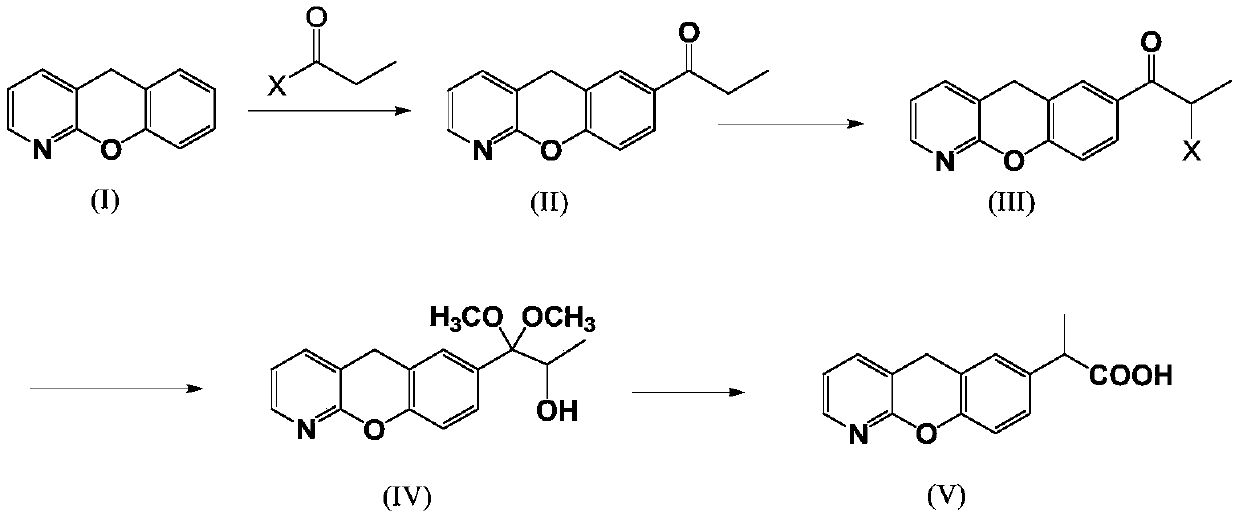
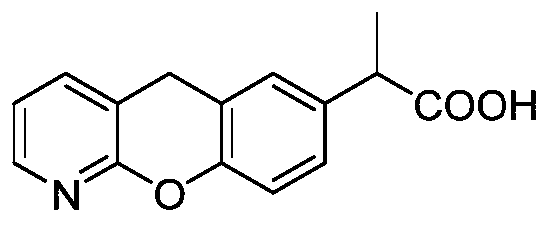
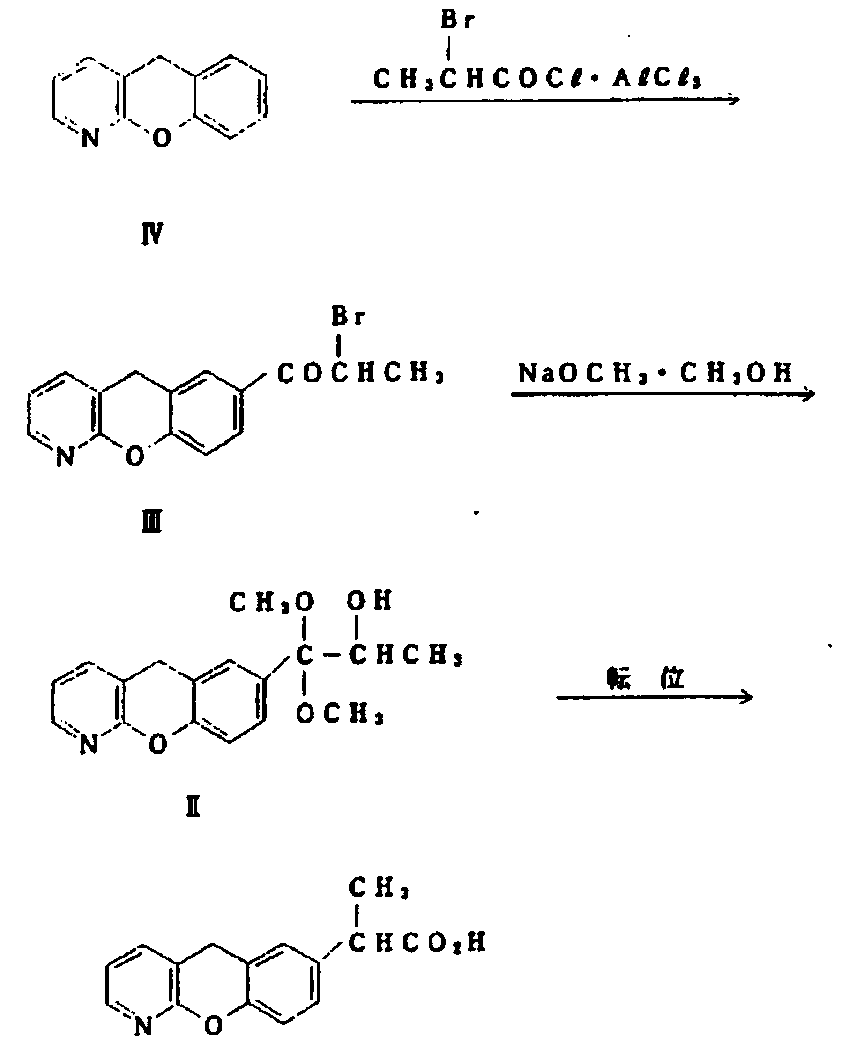
Novel preparation method of pranoprofen
The invention discloses a novel preparation method of pranoprofen. The novel preparation method of the pranoprofen has the advantages of being high in yield, having less impurities, and being safe andenvironmentally friendly. The novel preparation method of the pranoprofen comprises the following steps that step 1, 5H-[1]-benzopyran[2,3-b]pyridine and propionyl chloride are directly acylated; step 2, bromination is carried out; step 3, potassium tert-butoxide or sodium tert-butoxide is used for hydrolysis, and finally, rearrangement is carried out to obtain the pranoprofen.
Owner:GUANGDONG XIANQIANG PHARMA +2
Preparation method and intermediate of ramelteon
InactiveCN102924410AReduce dosageRaw materials are cheap and easy to getOrganic chemistryBulk chemical productionPropionyl chlorideOrganic solvent
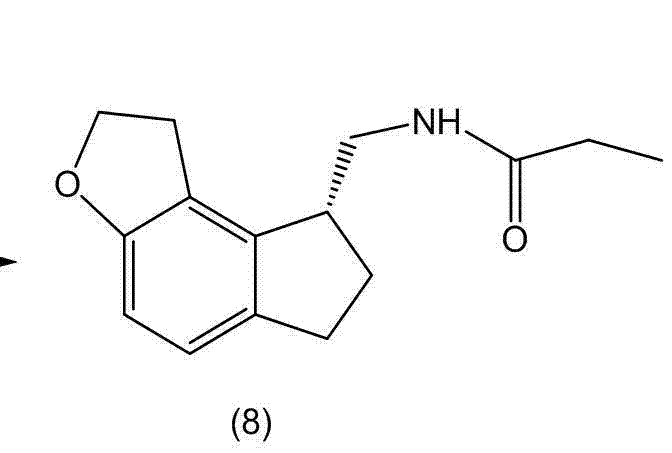
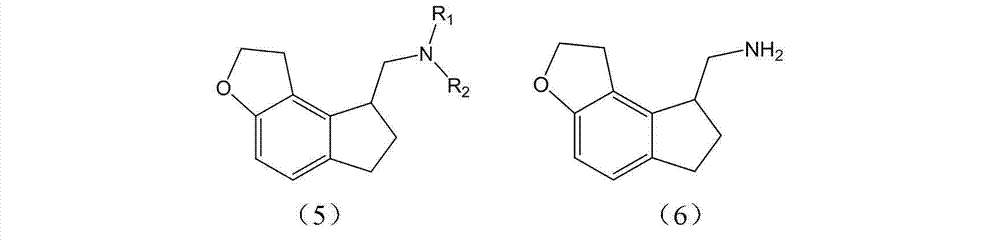
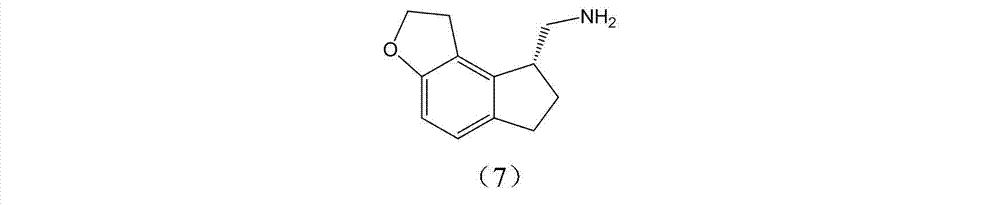
The invention discloses a preparation method and an intermediate of ramelteon. The preparation method comprises the following steps: 1. deprotecting a compound (5) in an organic solvent to obtain a compound (6); 2. after condensing the compound (6) and a chiral resolution reagent, crystallizing to obtain an S type condensate crystal, and hydrolyzing under alkaline conditions to obtain an S type optical isomer compound (7) of the compound (6); and 3. reacting the compound (7) with propionyl chloride under alkaline conditions to generate the ramelteon (8).
Owner:CHINA RESOURCES SAIKE PHARMA
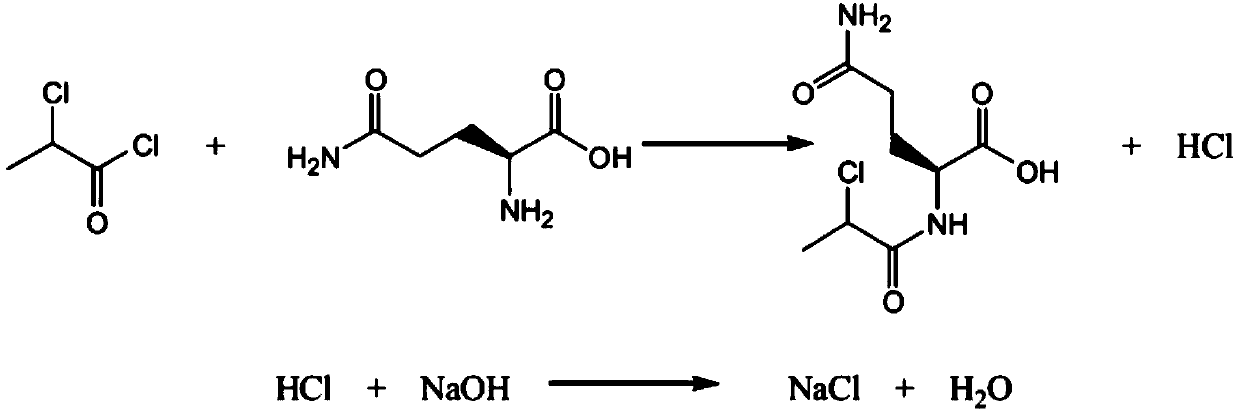
Synthesis method of N-(2)-L-alanyl-L-glutamine
Belonging to preparation of immune dipeptide compounds, the invention discloses a synthesis method of N(2)-L-alanyl-L-glutamine. The preparation steps include: taking D-2-chloropropanoyl chloride and L-glutamine as raw materials, conducting condensation to obtain N-(D-2-propionyl chloride)-L-glutamine, then performing ammoniation to obtain N(2)-L-alanyl-L-glutamine, and carrying out refining so as to obtain a refined product.
Owner:QINGDAO VLAND BIOTECH INC
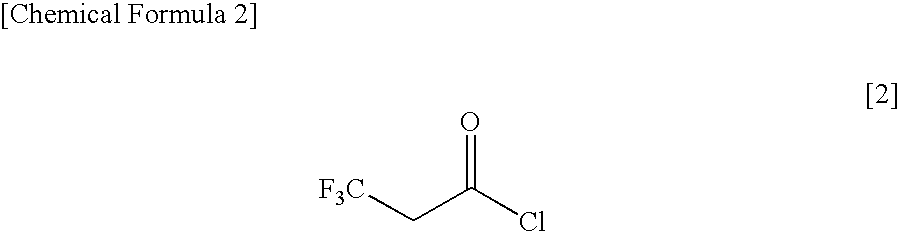
Method for Producing 3,3,3-Trifluoropropionyl Chloride
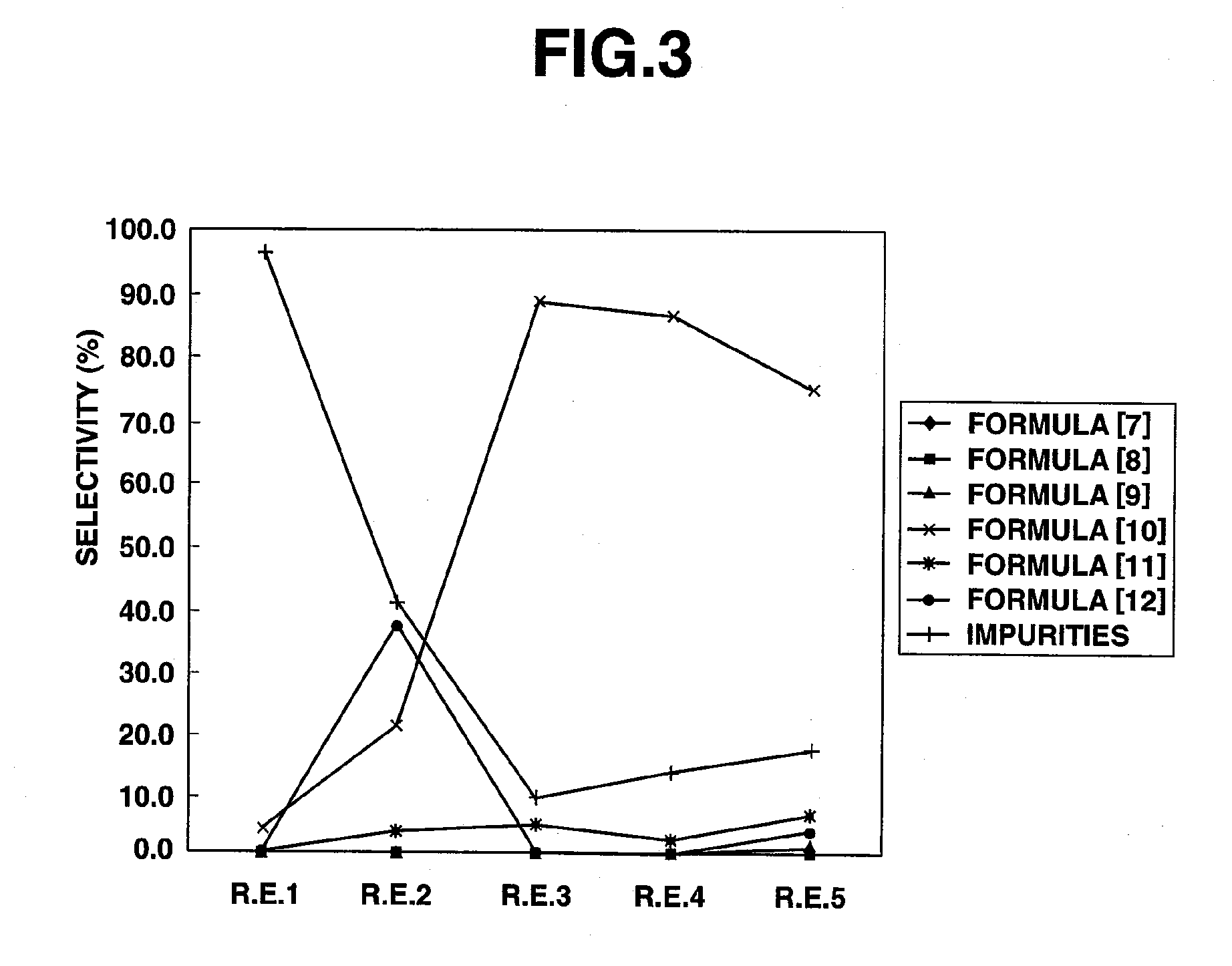
InactiveUS20100113834A1High selectivitySuppress generationOrganic compound preparationOxygen compounds preparation by reductionChlorideSulfuryl chloride
Owner:CENT GLASS CO LTD
Novel method for preparing N-phenyl-3-morpholine propanamide
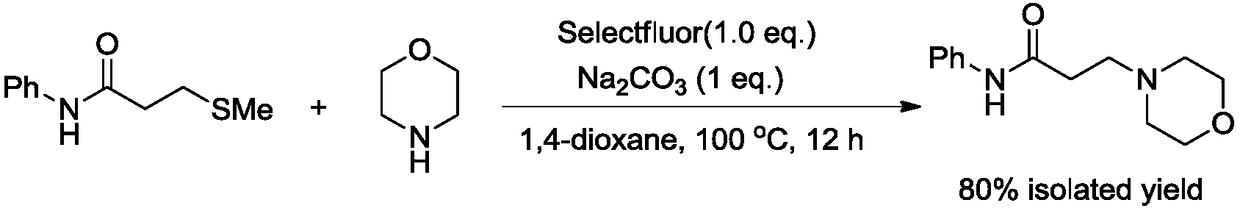
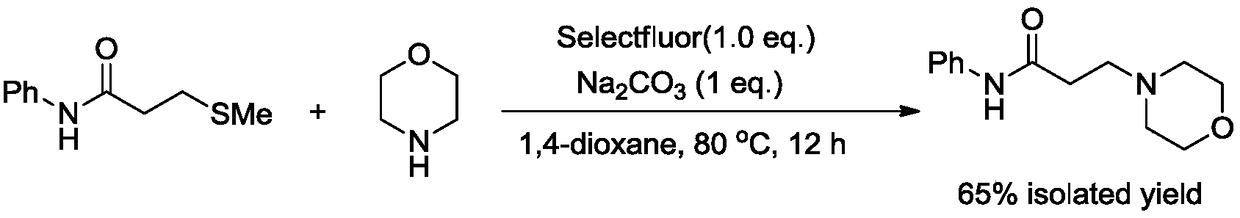
ActiveCN109232476AImprove efficiencySimple and fast operationOrganic chemistryTetrafluoroborateOrganic solvent
The invention relates to the technical field of fine chemical engineering, and discloses a novel method for preparing N-phenyl-3-morpholine propanamide. The method specifically comprises the followingstep that N-phenyl-3-methylthio propionamide, morpholine, 1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) and sodium carbonate undergo stirring reaction in an organicsolvent of 1,4-dioxane at the temperature of 100 DEG C for 12 hours so as to obtain the N-phenyl-3-morpholine propanamide. Compared with the prior art, the method has the advantages of being simple tooperate, rapid and efficient, in addition, toxic, perishable and volatile 3-chloropropionyl chloride does not need to be used as raw materials, and the method is environmentally friendly.
Owner:CHANGZHOU UNIV
Preparation method of agomelatine intermediate
InactiveCN114394906AShort reaction stepsMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationPropanoic acidReaction temperature
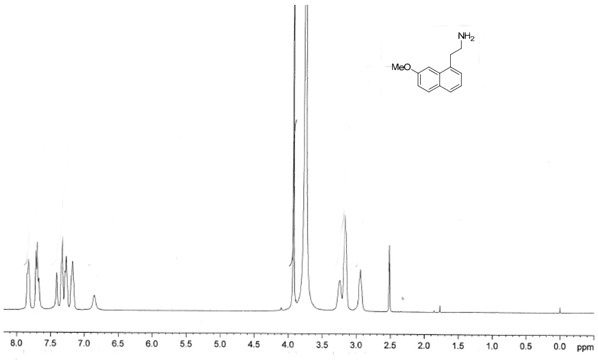
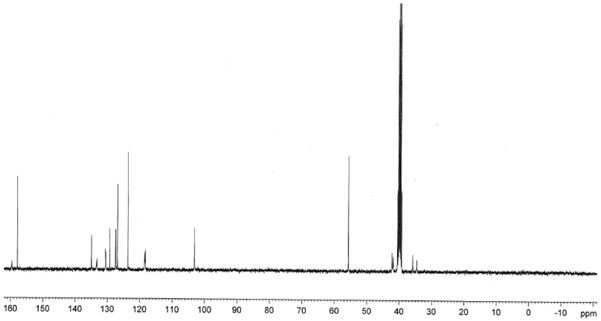
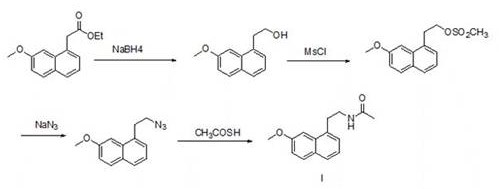
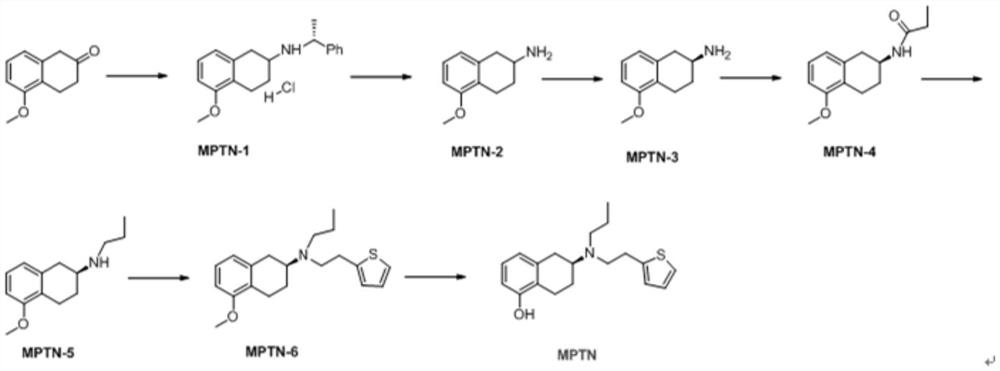
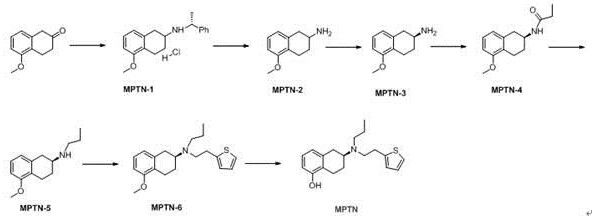
The invention relates to a preparation method of an agomelatine intermediate, which belongs to the field of drug intermediates, and comprises the following four steps: acylating chlorination and amidation of 7-methoxy-1-naphthyl propionic acid, Hofmann degradation of 7-methoxynaphthalene-1-yl propanamide, and post-treatment. 7-methoxy-1-naphthyl propionyl chloride obtained by carrying out acylating chlorination on the 7-methoxy-1-naphthyl propionic acid is subjected to amidation to obtain 7-methoxy-1-naphthyl propionamide, and then the 7-methoxy-1-naphthyl propionamide is subjected to Hofmann degradation to obtain the agomelatine intermediate 7-methoxy-1-naphthyl ethylamine. The preparation method disclosed by the invention is short in reaction step and mild in reaction condition, the reaction temperature is-5 to 100 DEG C, and the reaction pressure is normal pressure; an azide is not used, Raney nickel catalysis or palladium carbon catalytic hydrogenation operation is not involved, and the reaction operation is simple and safe; the total yield of the 7-methoxy-1-naphthyl ethylamine can reach 67.1%-78.4%, and the liquid phase purity can reach 98.1%-98.9%.
Owner:SHANDONG WEIFANG PHARMA FACTORY
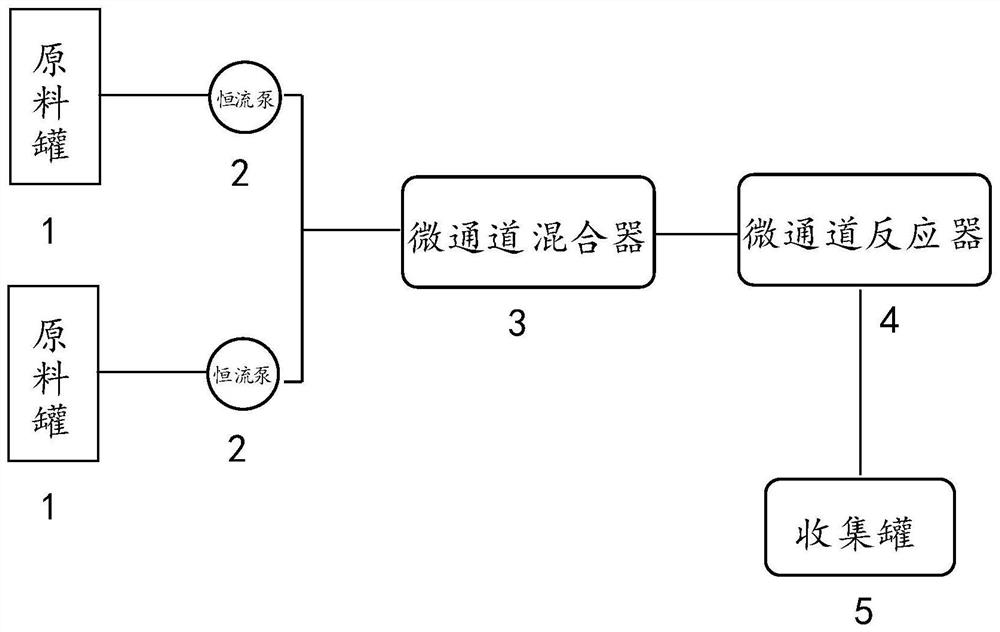
Method for preparing D-(+)-2-chloropropionyl chloride by adopting microchannel continuous flow reactor
PendingCN113603582AHigh control precisionReact SafeOrganic compound preparationOrganic chemistry methodsPtru catalystDistillation
The invention is applicable to the technical field of organic intermediate preparation processes, and provides a method for preparing D-(+)-2-chloropropionyl chloride by adopting a microchannel continuous flow reactor, wherein the method comprises the steps: dissolving L-lactic acid and a catalyst 2,6-di-tert-butylpyridine in dichloromethane to obtain a homogeneous solution A; dissolving thionyl chloride in dichloromethane to obtain a homogeneous solution B; respectively and simultaneously pumping the homogeneous solution A and the homogeneous solution B into a micro-mixer in a micro-channel continuous flow reaction device; introducing a mixed solution of the micro-mixer in the micro-channel continuous flow reaction device into a micro-reactor; and collecting the effluent of the micro-reactor, recycling a solvent by normal pressure distillation, and carrying out reduced pressure distillation to obtain the D-(+)-2-chloropropionyl chloride with optical purity. The micro-channel continuous flow reactor used in the method is in full-automatic formation production, the control precision is high, the reaction process is safe and controllable, and the obtained D-(+)-2-chloropropionyl chloride is high in yield and high in optical purity; and the mass and heat transfer performance is good, the D-(+)-2-chloropropionyl chloride can be continuously prepared on a large scale, and the application prospect is wide.
Owner:苏州永诺泓泽生物科技有限公司
Novel process for preparing chloropropionyl chloride
InactiveCN110950754AGood catalyticEasy to handlePhysical/chemical process catalystsOrganic compound preparationPhosphorous acidPropanoic acid
The invention discloses a novel process for preparing chloropropionyl chloride, which can overcome the defects that the byproduct phosphorous acid in the existing chloropropionyl chloride production process is not thoroughly separated and is not easy to treat and store; catalyst recycling is impossible to realize; the yield is low; the cost is high, and the like. According to the novel process, inthe acylation process, the product of the reaction of propionic acid and phosphorus trichloride is subjected to standing separation, the lower phosphorous acid is subjected to hydrolysis, decoloration, negative pressure distillation and slicing deep processing to reach the industrial standard of industrial phosphorous acid, the product at solid state is easy to store, byproducts are easy to sell,and extra economic benefits are brought to companies. A novel catalyst is used in the chlorination process of crude propionyl chloride at the upper layer, the catalyst is ideal in catalytic effect, can be repeatedly used, is not easy to carbonize at high temperature and has few residues, so that the quantity and the quality of products are improved.
Owner:XINHUA PHARM (SHOUGUANG) CO LTD
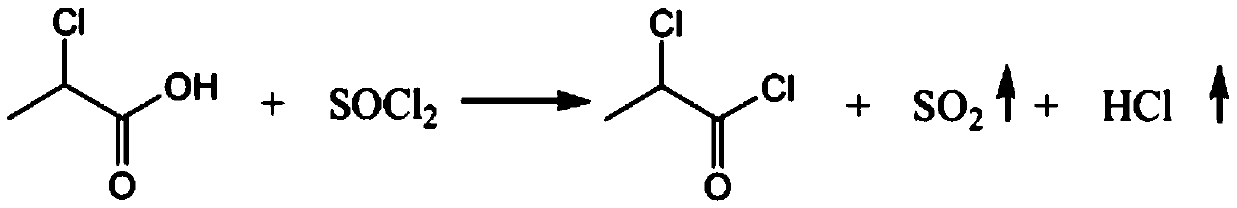
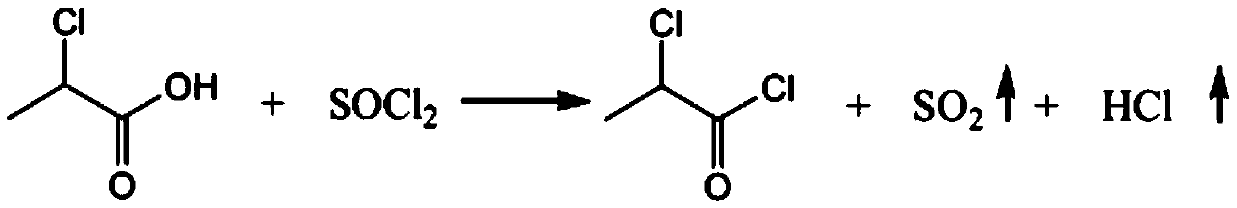
Production process of chloropropionyl glutamine
PendingCN110845351AIncrease profitSolve the troubleOrganic compound preparationOrganic chemistry methodsPropanoic acidPtru catalyst
The invention relates to the technical field of chemical engineering, and discloses a production process of chloropropionyl glutamine. The production process comprises following steps: carrying out acylating chlorination reaction: adopting D-2-chloropropionic acid and thionyl chloride as raw materials, adopting DMF (N, N-dimethylformamide) as a catalyst, and carrying out reaction to generate D-2-chloropropionyl chloride, sulfur dioxide and hydrogen chloride. According to the production process of chloropropionyl glutamine, 791kg of thionyl chloride is pressed into a dry and clean 2000 L enamelreaction kettle by using nitrogen, stirring is started, and 0.75kg of N, N-dimethylformamide is slowly dropwise added; then, 600kg of D-2-chloropropionic acid is pressed into a dry and clean 1000L enamel high-level tank by using nitrogen gas, a reaction kettle jacket is subjected to 75 DEG C hot water bath to increase the temperature of the system to 60-65 DEG C, D-2-chloropropionic acid is dropwise added into the reaction kettle for about 4 h at 60 to 65 DEG C, and the gas release amount is controlled by adjusting the dropwise adding speed. T production process is simple in steps, complete in reactant reaction, high in raw material utilization rate and low in preparation cost, troubles of users are avoided, and the production process is convenient for users to use.
Owner:ANHUI SHENGYUAN CHEM
Synthesis method of 3-chloropropionyl chloride
InactiveCN112409166ASimple processLess investment in equipmentOrganic compound preparationCarboxylic compound preparationPtru catalystDistillation
The invention discloses a synthesis method of 3-chloropropionyl chloride, and relates to the technical field of chemical engineering, which comprises the following steps: adding acrylic acid, a catalyst and water into a reactor, and conducting stirring; dropwise adding thionyl chloride under stirring while keeping the temperature at 20-80 DEG C; after dropwise adding thionyl chloride, conducting stirring for 0.5-2 hour at the temperature of 20-80 DEG C to obtain a crude product of 3-chloropropionyl chloride; and carrying out reduced pressure distillation on the crude product of 3-chloropropionyl chloride to obtain high-purity 3-chloropropionyl chloride. By adding the catalyst and water, the water and thionyl chloride generate extra sufficient hydrogen chloride in the reaction to make up for the loss of hydrogen chloride in the system, and the hydrogen chloride fully reacts with acrylic acid and acryloyl chloride under the action of the catalyst to generate 3-chloropropionic acid and 3-chloropropionyl chloride. The synthetic method has the advantages of simple process, less investment equipment, high equipment efficiency, easily available raw materials, low price and less generatedthree wastes, and the final purposes of high yield and high product content are achieved according to the synthetic method.
Owner:李雨
Preparation method of 3, 3, 3-trifluoropropionic acid
ActiveCN111039771AImprove conversion rateMild reaction conditionsPreparation from carboxylic acid halideOrganic compound preparationPropanoic acidHydrolysis
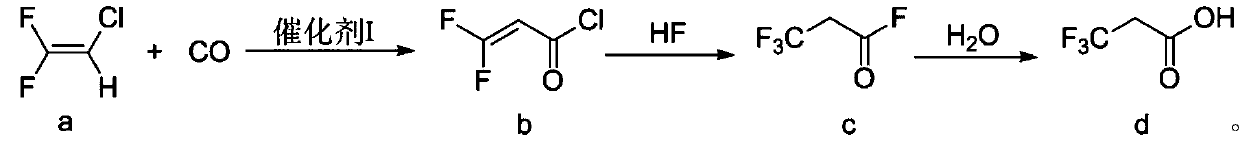
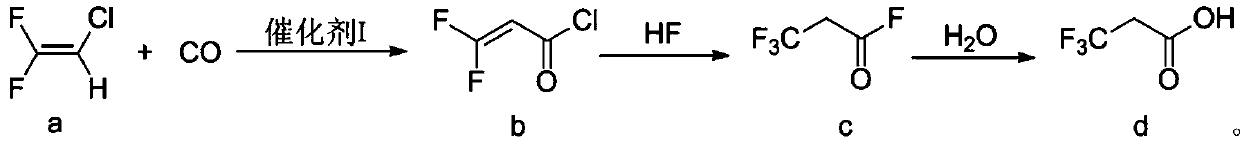
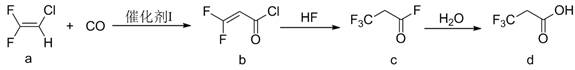
The invention discloses a preparation method of 3, 3, 3-trifluoropropionic acid. The preparation method comprises the following steps: firstly, taking 2-chloro-1-1-difluoroethylene (a) as a raw material and carrying out a carbonylation reaction between the raw material and carbon monoxide to obtain 3, 3-difluoro-2-allyl chloride (b); carrying out a fluorination reaction on 3, 3-difluoro 2-allyl chloride by using hydrogen fluoride (HF) to obtain 3, 3, 3-trifluoropropionyl fluoride (c); and finally hydrolyzing the 3, 3, 3-trifluoropropionyl fluoride to obtain trifluoropropionic acid (d). The preparation method has the advantages as follows: the raw materials are cheap and easily available; conditions are mild; conversion rate of the starting material is high; and the total yield of the three-step reactions can reach 50% or above.
Owner:湖南有色郴州氟化学有限公司
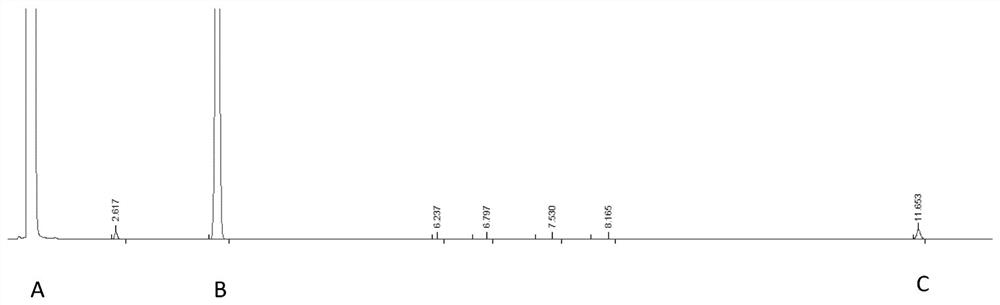
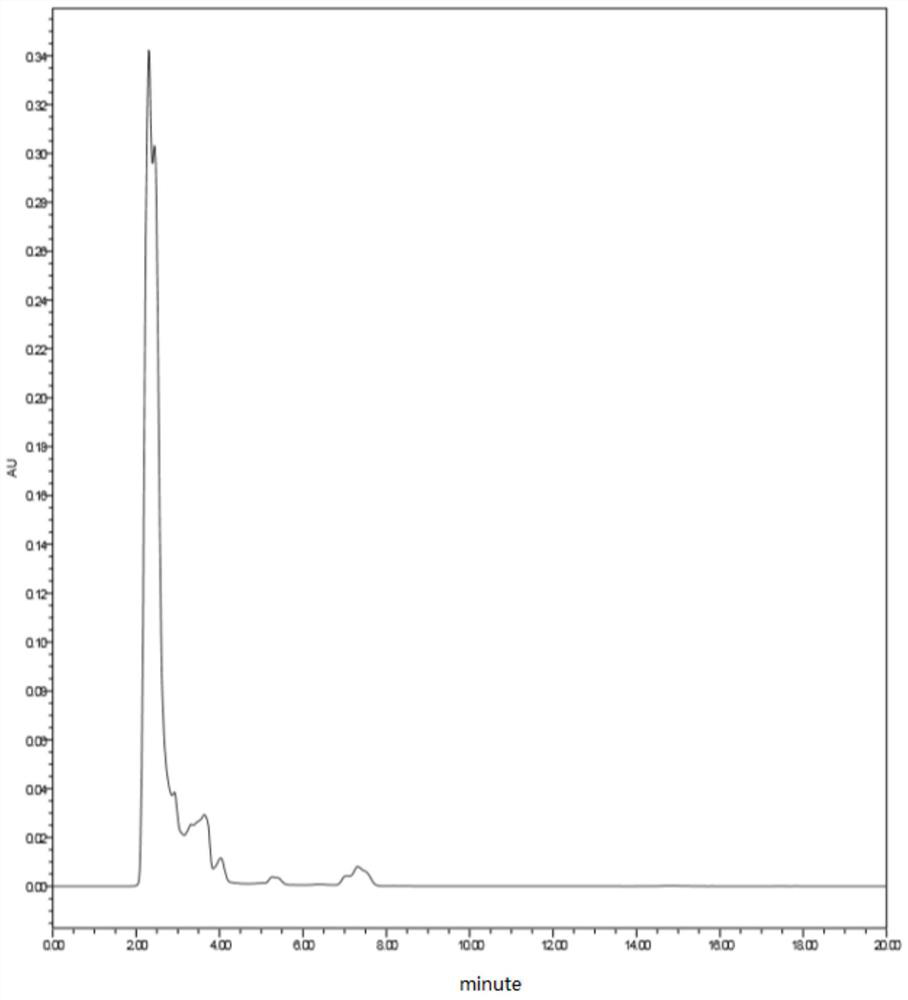
Gas chromatographic analysis method of 3-chloropropionyl chloride
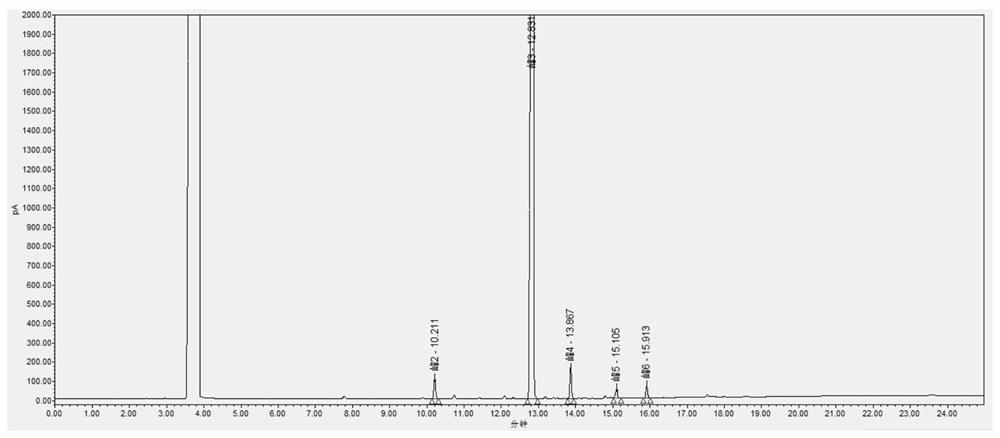
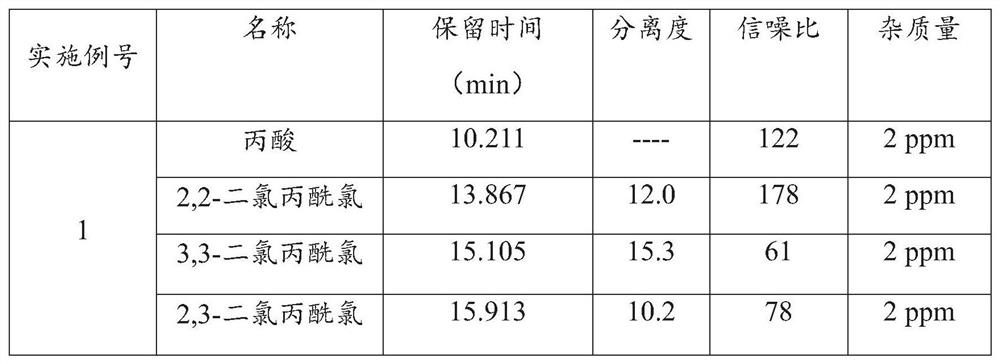
InactiveCN113030288AExtended service lifeAccurate detectionComponent separationPropanoic acidGas liquid chromatographic
The invention provides a gas chromatographic analysis method of 3-chloropropionyl chloride. The method comprises the following steps of: taking a 3-chloropropionyl chloride sample in a container; adding methanol to realize esterification, and shaking up the container; introducing a sample after derivatization; separating and detecting the introduced sample through a gas chromatograph to obtain a gas chromatogram; and calculating the content percentage of the 3-chloropropionyl chloride through an area normalization method. According to the method of the present invention, an FFAP capillary chromatographic column is adopted, a result obtained through the detection of an FID detector is analyzed through the area normalization method, and therefore, the detection speed is fast, the result is accurate, the method is simple and practical; the 3-chloropropionic acid content and the 3-chloropropionyl chloride content in a prepared 3-chloropropionyl chloride product can be accurately detected; and the service life of the chromatographic column can be effectively prolonged.
Owner:NANTONG TENDENCI CHEM
Synthesis method of chickpea dentin A
The invention provides a synthesis method of chickpea dentin A, which solves the problems of incomplete reaction, easy caking of a reaction system and low yield due to use of reagents with high risk during synthesis of chickpea dentin A in the prior art. The preparation method comprises the following steps of introducing dry hydrogen chloride gas into anhydrous phloroglucinol and p-methoxyphenylacetonitrile in ethyl acetate to carry out Houben-Hoesch condensation reaction, hydrolyzing the condensation product to obtain an intermediate, and cyclizing the intermediate in acetone by propionyl chloride and sodium formate to obtain the chickpea dentin A.
Owner:SHAANXI JIAHE PHYTOCHEM
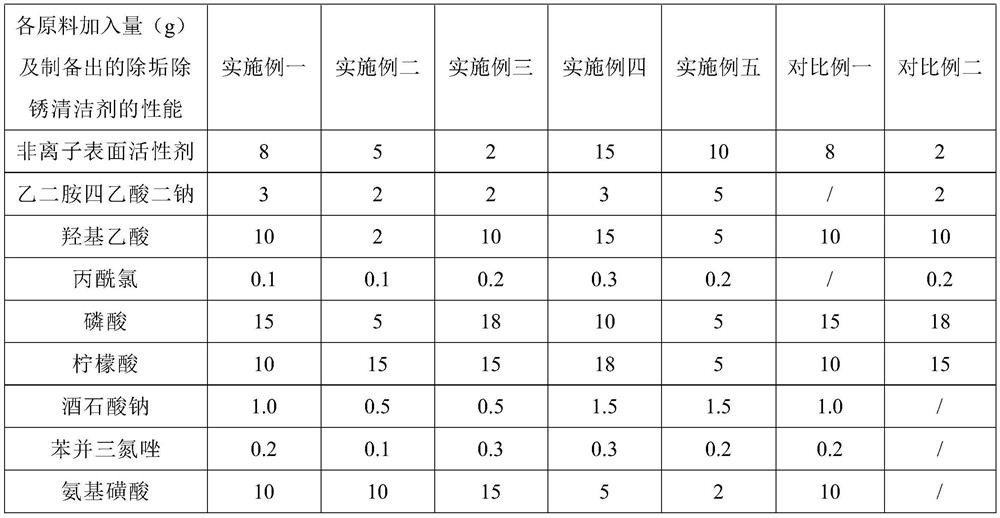
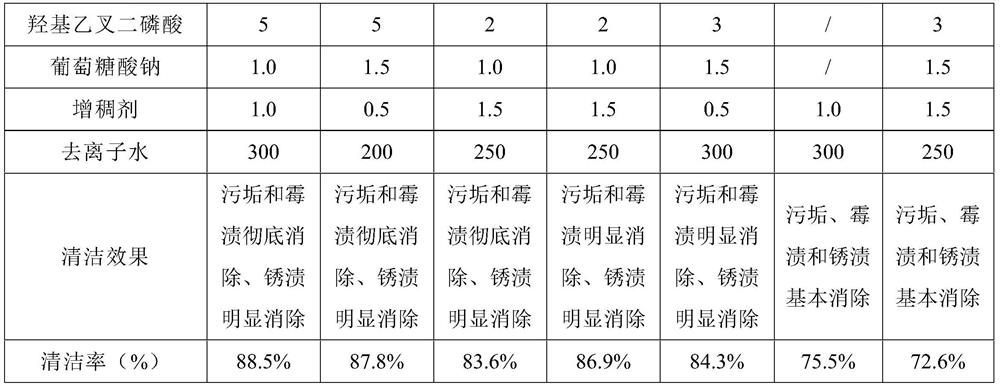
Gel-like descaling and derusting cleaning agent and preparation method thereof
InactiveCN112226294AEasy to cleanSo as not to damageInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsO-Phosphoric AcidActive agent
The invention provides a gel-like descaling and derusting cleaning agent, which is prepared from the following raw materials in parts by weight: 2 to 15 parts of nonionic surfactant, 2 to 5 parts of disodium ethylene diamine tetraacetate, 2 to 15 parts of glycolic acid, 0.1 to 0.3 part of propionyl chloride, 5 to 18 parts of phosphoric acid, 5 to 18 parts of citric acid, 0.5 to 1.5 parts of sodiumtartrate, 0.1 to 0.3 part of benzotriazole, 2 to 15 parts of sulfamic acid, 0.5 to 1.5 parts of a thickening agent, 2 to 5 parts of hydroxyethylidene diphosphate, 0.5 to 1.5 parts of sodium gluconateand 200 to 350 parts of deionized water. The gel-like descaling and derusting cleaning agent has the beneficial effects of being good in cleaning effect and not prone to damaging utensils, and is suitable for the field of cleaning agents.
Owner:山西洁锐奇化工有限公司
Lignin based antioxidant for polyolefin, preparation method and application
The invention relates to the technical field of antioxidants, in particular to a lignin based antioxidant for polyolefin, a preparation method and an application. The lignin based antioxidant is prepared from the following raw materials in parts by weight: 100 parts of industrial lignin, 50-200 parts of a modified monomer, 3-10 parts of an organic basic catalyst and 500 parts of a solvent, wherein the modified monomer is 3,5-di-tert-butyl-4-hydroxyphenylpropionyl chloride. The preparation method comprises the following steps: (1) stirring and mixing the industrial lignin and the solvent uniformly; (2) then, adding the modified monomer and the organic basic catalyst in sequence for a reaction for 12-72 h at 40-80 DEG C, precipitating the product with a precipitant, and performing suction filtration, washing and drying to obtain the lignin based antioxidant for polyolefin. The industrial lignin is from recycled pulping and papermaking waste liquor and the bioethanol industry, is low in price and renewable; the preparation method is simple, the cost is low, the prepared lignin based antioxidant has outstanding performance and is of great significance in promoting application and development of polyolefin and high value-added utilization of the industrial lignin.
Owner:WUHAN TEXTILE UNIV
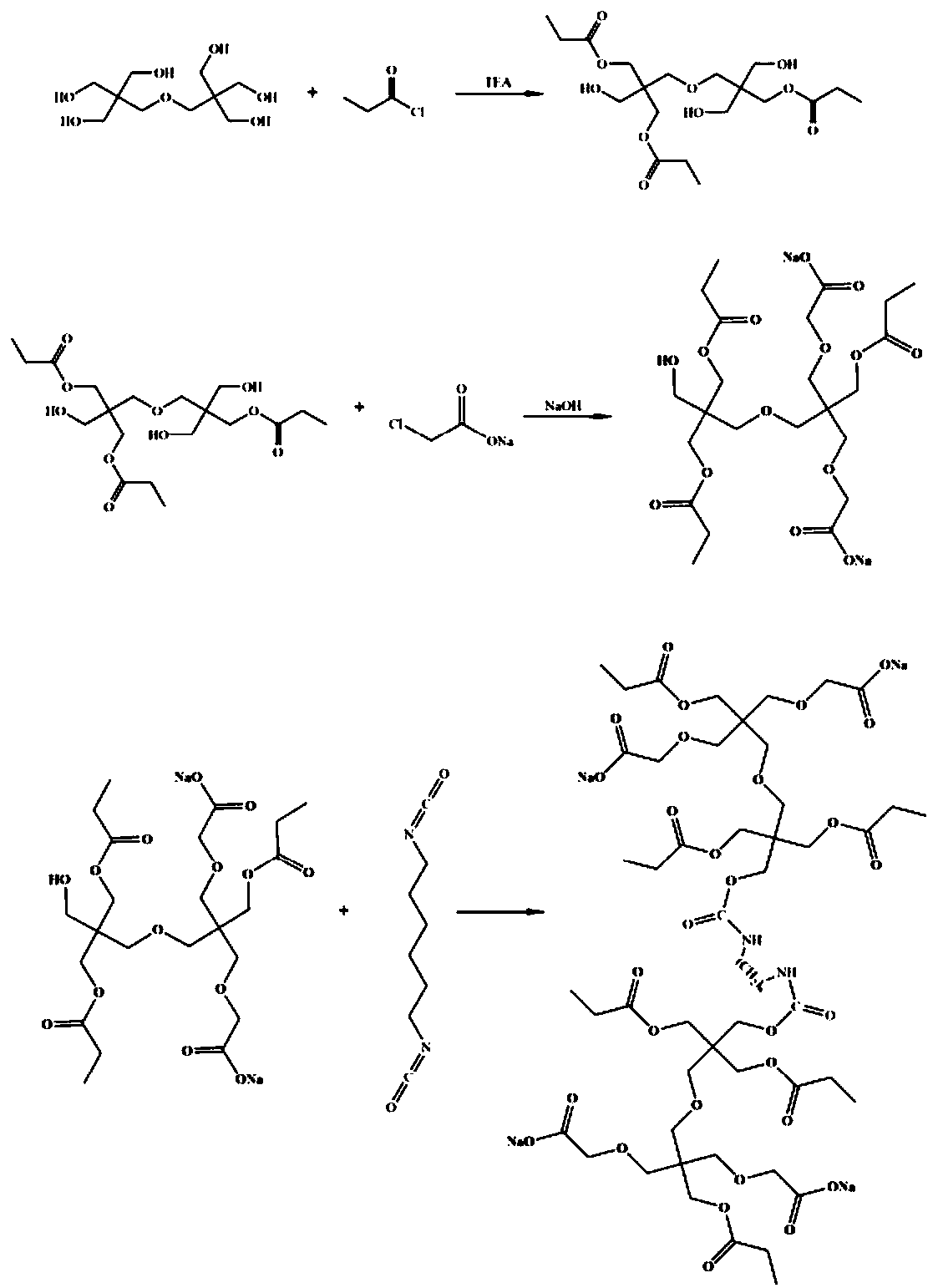
Multi-carboxyl chelating agent as well as preparation method and application thereof
ActiveCN111574406AImprove solubilityReduce usagePreparation from carboxylic acid halidesCarbamic acid derivatives preparationFlocculationSodium chloroacetate
The invention discloses a multi-carboxyl chelating agent as well as a preparation method and application thereof. The preparation method comprises the following steps: (1) adding dipentaerythritol andpropionyl chloride into a three-neck flask to obtain a viscous liquid intermediate I; (2) adding the viscous liquid intermediate I obtained in the step (1) into a four-neck flask, then adding a firstsolvent dimethyl sulfoxide into the four-neck flask, and then adding sodium chloroacetate into a solution containing solvents such as dimethyl sulfoxide to obtain a solid product intermediate II; and(3) adding the solid product intermediate II in the step (2) into a second solvent dimethylformamide, and adding hexamethylene diisocyanate (HDI) under magnetic stirring to obtain the multi-carboxylchelating agent. According to the preparation method of the multi-carboxyl chelating agent, the heavy metal chelating agent with dual functions of flocculation and chelating is successfully prepared.
Owner:SHAANXI UNIV OF SCI & TECH
Preparation process of duloxetine
Owner:武汉励合生物医药科技有限公司
Nano lamina compound with regular arranged amino radicles
The invention relates to a nanometer layer structure compound and the manufacturing method. It uses 3-amino propyl-3- methoxyl group silicane and amphi-amino benzene 3- methoxy silicane as structure forming material, using the NH2 radical to form layer structure compound under acid condition, and using chloracetyl chloride, 3- chlorine propionyl chloride, 4-chlorine butyric acid chloride, 5-chlorine valeryl chlorine, parachlorobenzoyl chloride, para-chlorobenzene acetyl chloride and the amido and gaining the nanometer layer structure compound with stable and ruled chlorine radical.
Owner:SHANGHAI UNIV
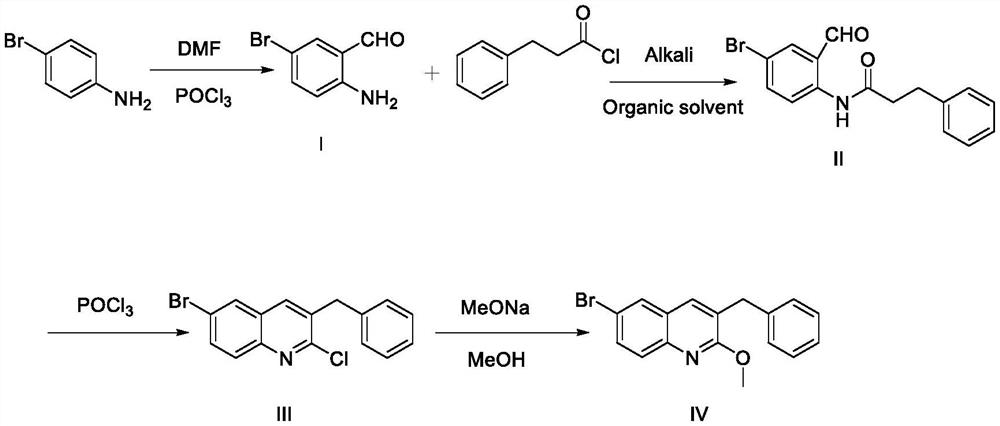
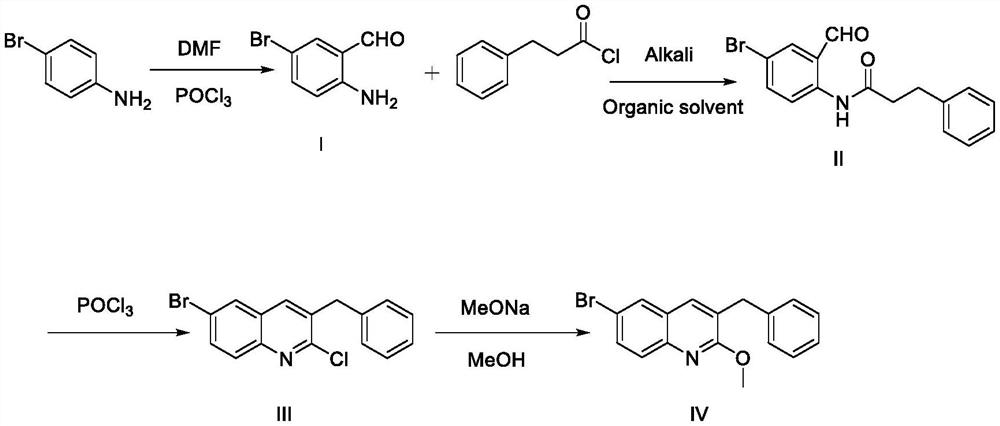
Synthesis method of 3-benzyl-6-bromo-2-methoxyquinoline
PendingCN114835641AThe reaction process is clearHigh yieldOrganic chemistrySodium methoxideQuinoline
The invention relates to the technical field of medicine synthesis, in particular to a synthesis method of 3-benzyl-6-bromo-2-methoxyquinoline. The synthesis method comprises the following steps: step 1, reacting p-bromoaniline with phosphorus oxychloride and N, N-dimethylformamide to generate a compound I; step 2, enabling the compound I to react with benzene propionyl chloride to generate N-(4-bromine-2-formyl phenyl)-3-benzene propionamide; 3, reacting the compound II with phosphorus oxychloride to generate a compound III; 4, reacting the compound III with sodium methoxide to generate a compound IV; the reaction process is clearer, and the yield is obviously improved compared with that of the original process; according to the Vilsmeier-Haack provided by the invention, only one aldehyde group is needed, and meanwhile, a solvent is added to participate in the reaction process, so that the problems of insufficient reaction, violent heat release and the like which are not beneficial to large-scale production operation caused by a curing phenomenon in the early-stage reaction of phosphorus oxychloride and N, N-dimethylformamide are avoided.
Owner:NANJING JIEYUN PHARMA TECH CO LTD
A kind of preparation method of rotigotine
ActiveCN113234057BReduce lossHigh split yieldOrganic compound preparationOrganic chemistry methodsTetraloneMandelic acid
Owner:CHENGDU TECH UNIV +1
A kind of preparation method of 3,3,3-trifluoropropionic acid
ActiveCN111039771BImprove conversion rateMild reaction conditionsPreparation from carboxylic acid halideOrganic compound preparationPropanoic acidPropanoyl chloride
Owner:湖南有色郴州氟化学有限公司
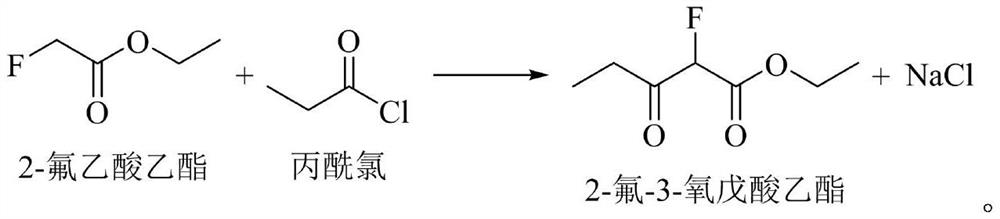
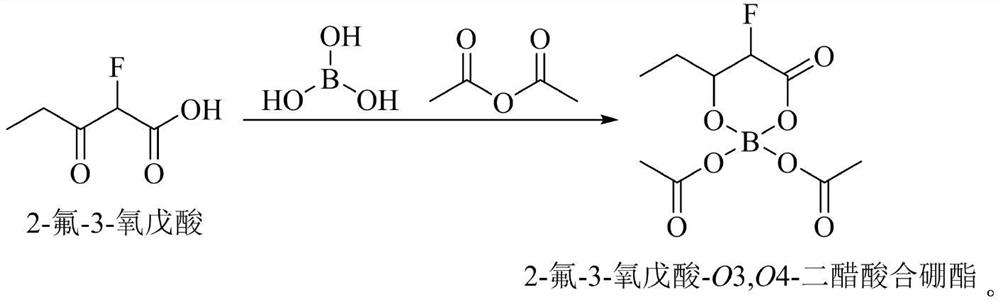
Method for continuously preparing voriconazole intermediate 2-fluoro-3-oxopentanoic acid ethyl ester
ActiveCN110903194BLow boiling pointReduce energy consumptionOrganic compound preparationCarboxylic acid esters separation/purificationFluoroacetic acidEthylic acid
The invention discloses a method for continuously preparing voriconazole intermediate 2-fluoro-3-oxyvaleric acid ethyl ester, comprising: feeding a reaction solvent, 2-fluoro-ethyl acetate, propionyl chloride and sodium hydrogen into a reaction kettle for reaction, Then add boric acid and acid anhydride, then send the reaction solution into the centrifuge, the supernatant is continuously sent to the middle of the rectification tower for heating and rectification, the light components are condensed at the top of the tower, and refluxed into the reaction kettle, and the heavy component products are extracted from the tower Bottom collection, that is. The invention realizes the continuous production process of the reaction of 2-fluoro-ethyl acetate and propionyl chloride and the separation of 2-fluoro-3-oxopentanoic acid ethyl ester through the reaction kettle coupling rectification tower. By adding boric acid and anhydride after the reaction, the reaction by-product 2-fluoro-3-oxopentanoic acid is converted into 2-fluoro-3-oxovaleric acid- which is easily separated from the main product O 3, O 4‑boryl diacetate improves the purity of the product, realizes low energy consumption, simple and rapid continuous production, the product yield is greater than 90%, and the purity is above 98%.
Owner:南京恒道医药科技股份有限公司
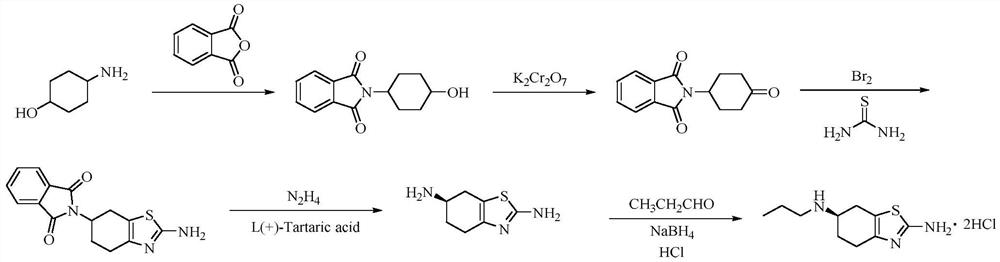
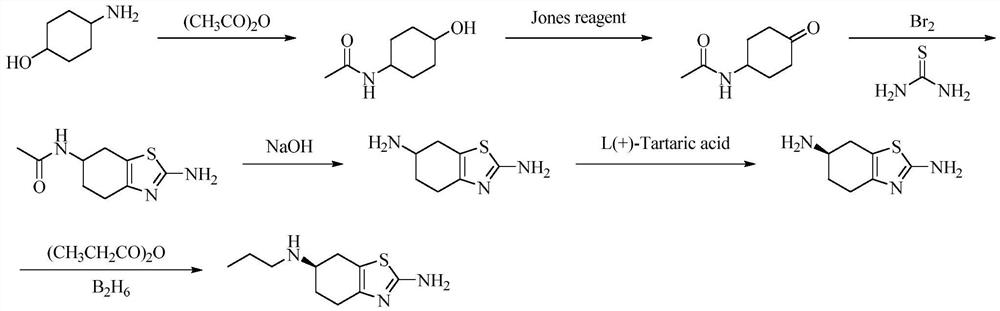
A kind of preparation method of pramipexole hydrochloride
ActiveCN109232471BAvoid lostRaw materials are cheap and easy to getOrganic chemistryCyclohexanoneCatalytic oxidation
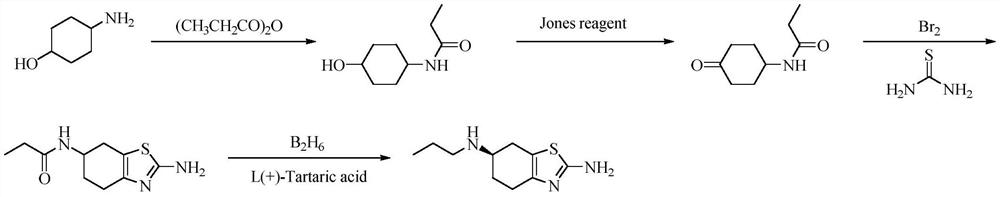
The invention discloses a preparation method of pramipexole hydrochloride, which comprises the following steps: p-aminocyclohexanol is condensed and protected by propionyl chloride to obtain p-propionyl cyclohexanol, and then catalyzed to obtain p-propionyl cyclohexanone, and then Oxidative bromination and Hantzsch condensation to give 2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole, and reduction with diisobutylaluminum hydride to give 2-amino-6-propylamino ‑4, 5, 6, 7‑tetrahydrobenzothiazole, finally separated by L‑(+)‑tartaric acid and salified to obtain pramipexole hydrochloride. The preparation method of pramipexole hydrochloride proposed by the invention has cheap and easy-to-obtain raw materials, convenient and simple operation, novel route, environmental protection, high yield, and the prepared pramipexole hydrochloride has good purity.
Owner:ANHUI QINGYUN PHARMA & CHEM
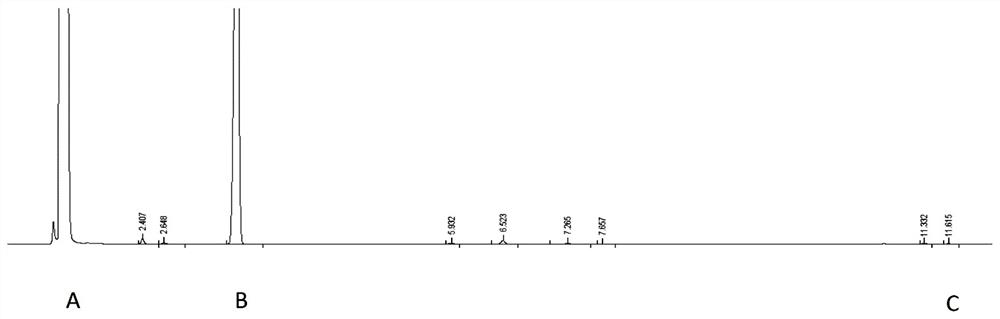
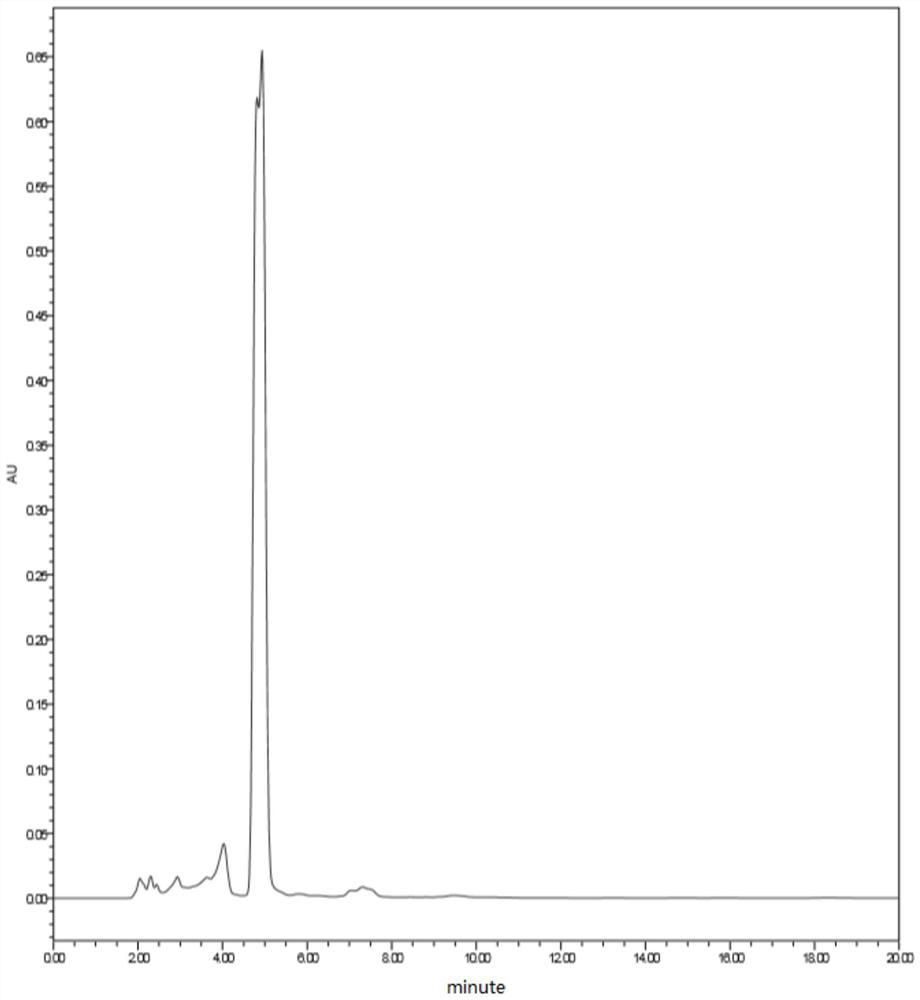
A method for effectively detecting the purity of 2-chloropropionyl chloride
ActiveCN110441446BAchieve derivationAvoid corrosionComponent separationAlcoholGas liquid chromatographic
The invention discloses a detection method for the purity of 2-chloropropionyl chloride, which utilizes the property that the acid chloride is easy to react with alcohols to form esters for derivatization treatment, avoiding the corrosion caused by the direct measurement of 2-chloropropionyl chloride and the inaccurate measurement results. Stable, then by gas chromatographic detection, the final area normalization method calculates 2-chloropropionyl chloride purity. The detection method can effectively separate the main component peak and the impurity peak of 2-chloropropionyl chloride, which greatly improves the detection sensitivity of 2-chloropropionyl chloride, so that the purity of 2-chloropropionyl chloride can be detected simply, quickly and stably. The detection method is simple in operation, high in accuracy, high in sensitivity and precision, good in stability and good in reproducibility.
Owner:HARVEST PHARMA HUNAN CO LTD
Continuous production method suitable for ibuprofen Friedel-Crafts reaction
ActiveCN114085135AImprove automationImprove securityCarbonyl compound preparation by condensationPropanoyl chlorideProcess engineering
The invention provides a continuous production method suitable for an ibuprofen Friedel-Crafts reaction, which is characterized in that a chloropropionyl chloride complexing solution and a Friedel-Crafts reaction circulating solution are uniformly mixed in a static mixer to obtain a mixed solution, the mixed solution and isobutylbenzene enter a jet reactor at the same time, the mixed solution is used as a jet reactor driving flow, and the isobutylbenzene is used as a jet reactor driven flow; a chloroketone solution generated by the Friedel-Crafts reaction is continuously discharged through a circulating pump; according to the invention, the continuous production of the ibuprofen Friedel-Crafts reaction is realized, the automatic control is easy to realize, the safety of the system is improved, the labor intensity is reduced, the reaction retention time is shortened, and the production efficiency is improved by more than 5-10 times.
Owner:SHANDONG XINHUA PHARMA CO LTD +2
Aging-resistant, waterproof and oil-resistant water-based ceramic-like paint and preparation method thereof
The invention relates to the field of architectural paint, in particular to aging-resistant, waterproof and oil-resistant water-based ceramic-like paint and a preparation method thereof. The aging-resistant, waterproof and oil-resistant water-based ceramic-like paint comprises the following raw materials of: in parts by weight, 26 parts of a modified polyimide resin solution, 12 parts of ash calcium powder, 8 parts of calcite powder, 7 parts of titanium dioxide, 3 parts of bentonite, 6.6 parts of aerogel thermal-insulating particles, 1.2 parts of nano aluminum oxide, 0.2 part of a water-basedantifoaming agent, 0.4 part of alkali-swelling thickener, 90 parts of water, 0.1-0.4 part of diethyl phenylethylmalonate, 0.5 part of tetramethylethylene glycol, 0.2 part of trihydroxymethyl aminomethane and 0.2 part of 3-cyclopentylpropionyl chloride synergist. The aging resistance, water resistance and oil resistance of the ceramic-like paint is improved significantly, and cracking and pulverization do not occur easily.
Owner:段宝荣
A kind of alectinib intermediate and the preparation method of alectinib
Owner:XINFA PHARMA
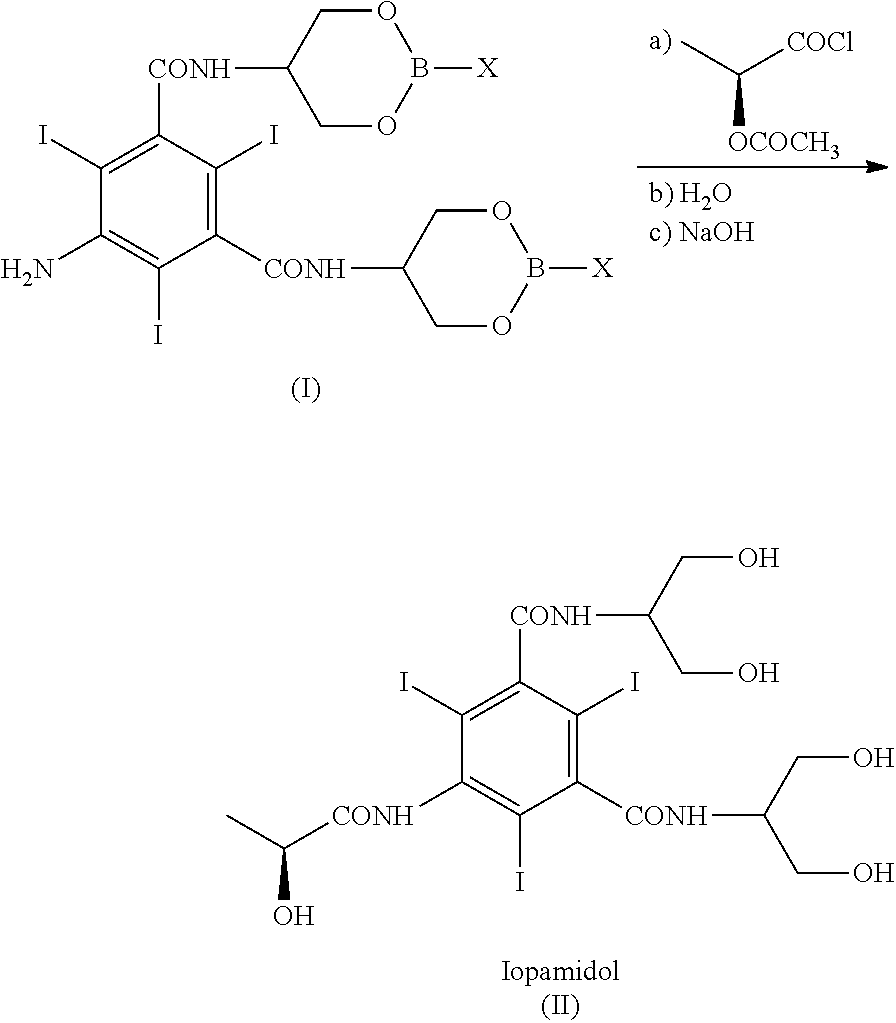
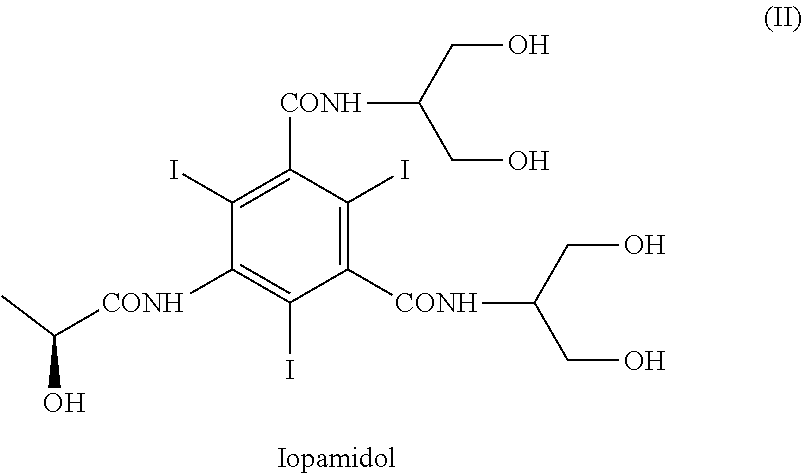
Process for the preparation of iopamidol
ActiveUS9950991B2Efficiently recyclableImprove processing yieldOrganic compound preparationCarboxylic acid amides preparationPotassium hydroxideAlkaline hydrolysis
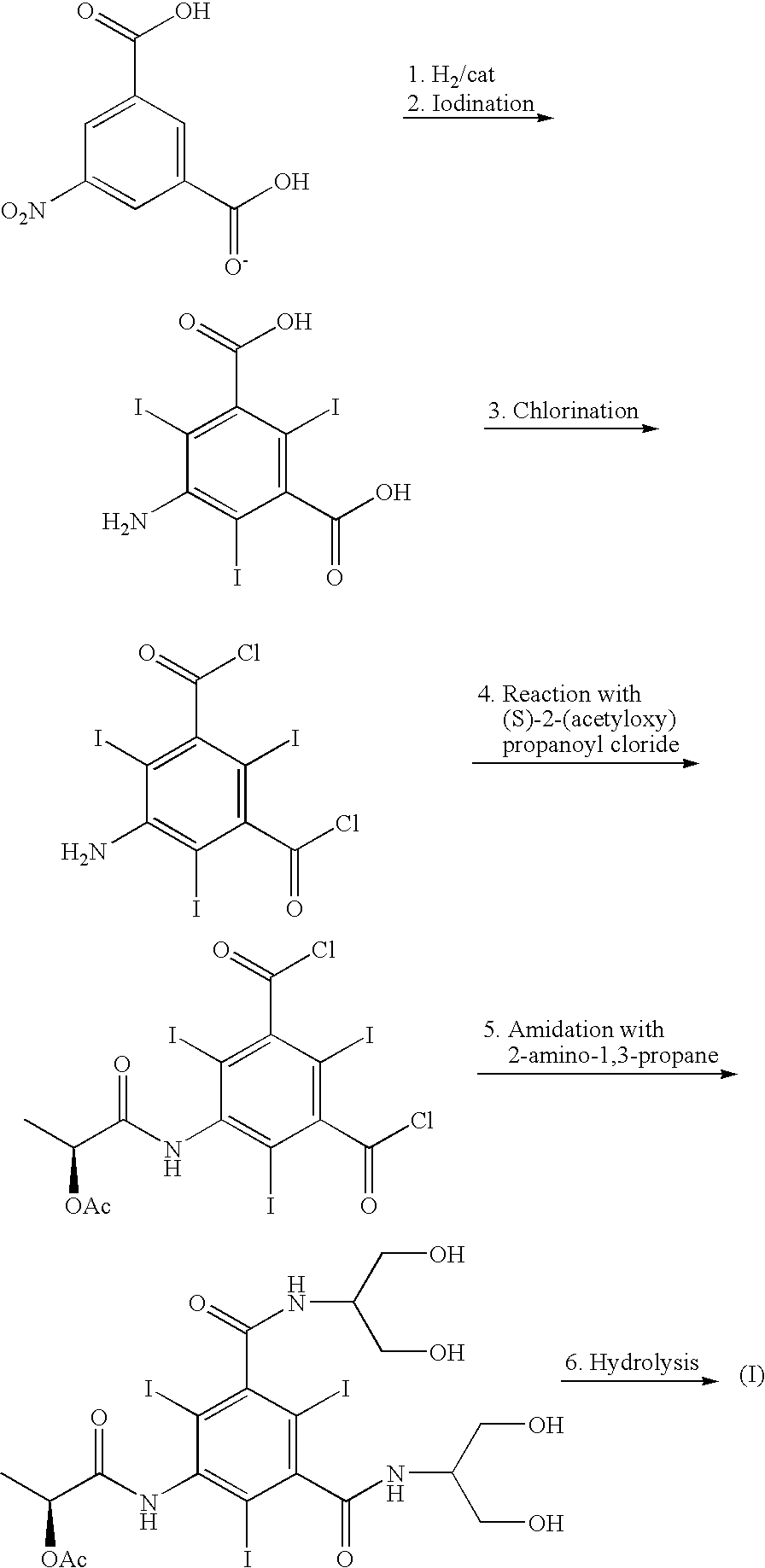
The present invention discloses a process for the preparation of Iopamidol of formula (II) and comprising the following steps: a) reacting the Compound (I) wherein X is OR2 or R3, and wherein R2 and R3 are a Ci-C6 linear or branched alkyl, C3-C6 cycloalkyl, C6 aryl, optionally substituted with a group selected from the group consisting of methyl, ethyl, n-propyl, i-propyl, n-butyl, sec-butyl, t-butyl and phenyl, with the acylating agent (S)-2-(acetyloxy)propanoyl chloride in a reaction medium to provide the acetyloxy derivative of Compound (I); b) hydrolyzing the intermediate from step a) with an aqueous solution at a pH comprised from 0 to 7, by adding water or a diluted alkaline solution such as sodium hydroxide or potassium hydroxide, freeing the hydroxyls from the boron-containing protective groups, obtaining the N—(S)-2-(acetyloxy)propanoyl derivative of Compound (II); c) alkaline hydrolysis to restore the (S)-2-(hydroxy)propanoyl group and to obtain Iopamidol (II) and optional recovery of the boron derivative from the solution obtained in step b). The boron-containing protective group is versatile, efficient and recyclable. A one-pot synthesis, without intermediate isolation is provided, leading to a decreasing of recovered and recycled solvents and a significant increasing in the yield, representing a significant advantage in terms of cost-effectiveness of the entire process and environmental awareness.
Owner:BRACCO IMAGINIG SPA
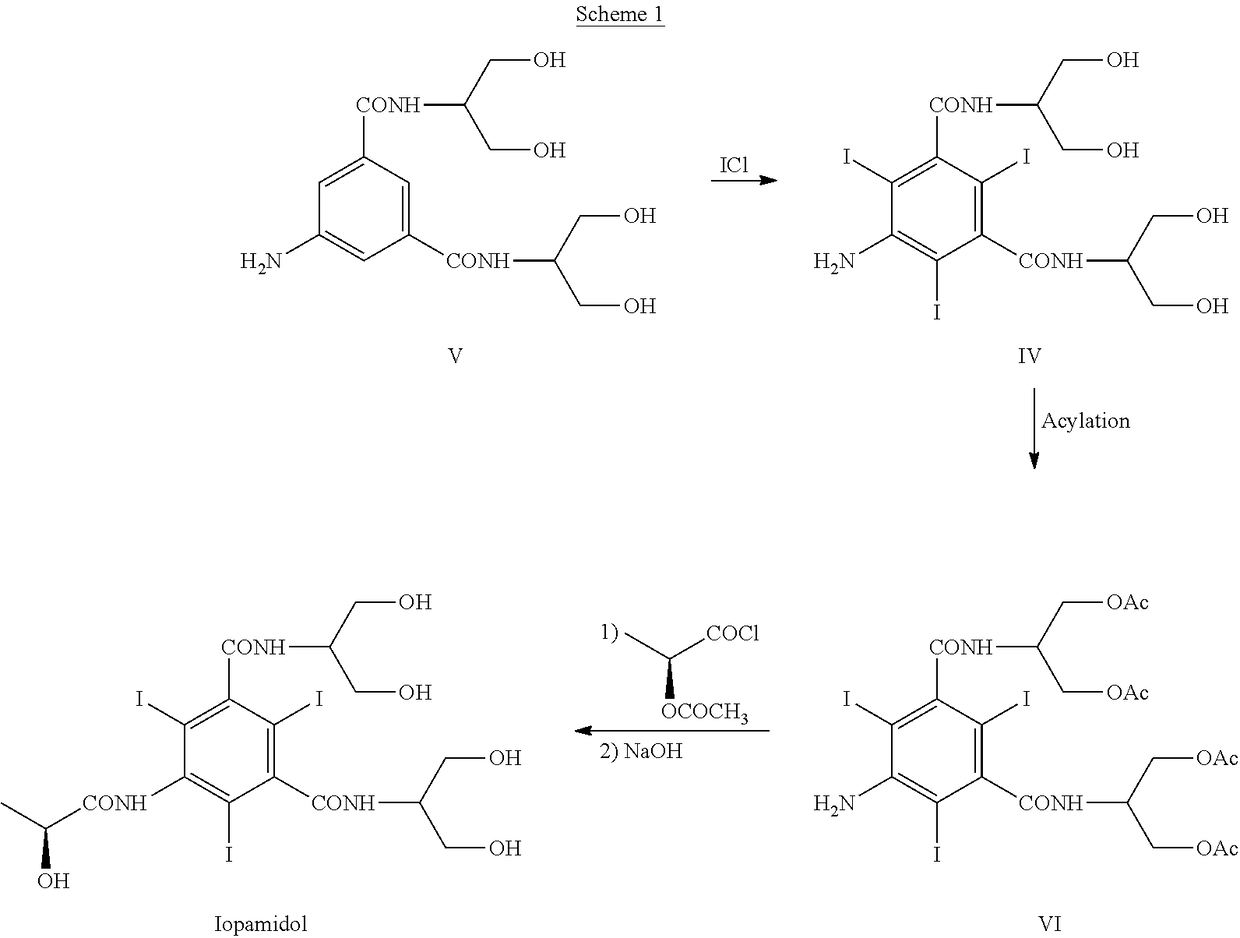
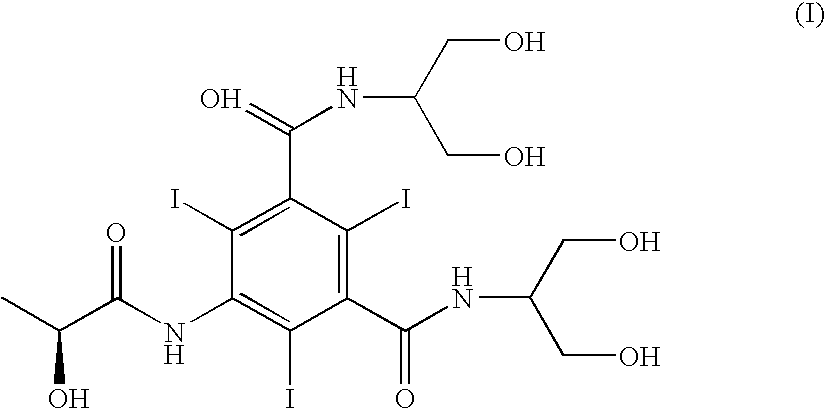
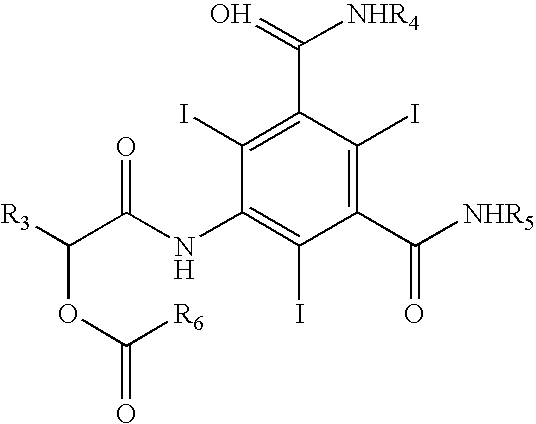
Process for the preparation of iopamidol and the new intermediates therein
InactiveUS20060106253A1High optical purityOrganic compound preparationOrganic chemistry methodsHydrogenAlkoxy group
A process for the preparation of (S)-N,N′-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-5-[(2-hydroxy-1-oxo-propyl)amino]-2,4,6-triiodo-1,3-benzendicarboxamide (iopamidol) starting from 5-amino-N,N′-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-2,4,6-triiodo-1,3-benzenedicarboxamide (II) which process comprises a) reacting the compound of formula (II) with a suitable protecting agent, to give a compound of formula (III) wherein R is a group of formula A or B wherein R1 is a hydrogen atom, a C1÷C4 straight or branched alkoxy group, R2 is hydrogen, a C1÷C4 straight or branched alkoxy group and R3 ?is a C1÷C4 straight or branched alkyl group, a trifuoromethyl or a trichloromethyl group; b) acylating the amino group in position 5 of the intermediate compound of formula (III), by reaction with a (S)-2-(acetyloxy)propanoyl chloride to give a compound of formula (IV) wherein R is as defined above; and c) removing all the acyl groups present in the compound of formula (IV) under basic conditions, with prior cleavage of the cyclic protections of the hydroxy groups in the carboxamido substituents under acidic conditions, when R is a group of formula A carboxamido hydroxy groups under acidic conditions. The invention also refers to the new intermediates of formula (III) and (IV) wherein —R is a group A.
Owner:BRACCO IMAGINIG SPA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com