Patents
Literature
52 results about "2-Chloropropionic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
2-Chloropropionic acid (2-chloropropanoic acid) is the chemical compound with the formula CH₃CHClCO₂H. This colorless liquid is the simplest chiral chlorocarboxylic acid, and it is noteworthy for being readily available as a single enantiomer. The conjugate base of 2-chloropropionic acid (CH₃CHClCO₂⁻), as well as its salts and esters, are known as 2-chloropropionates or 2-chloropropanoates.
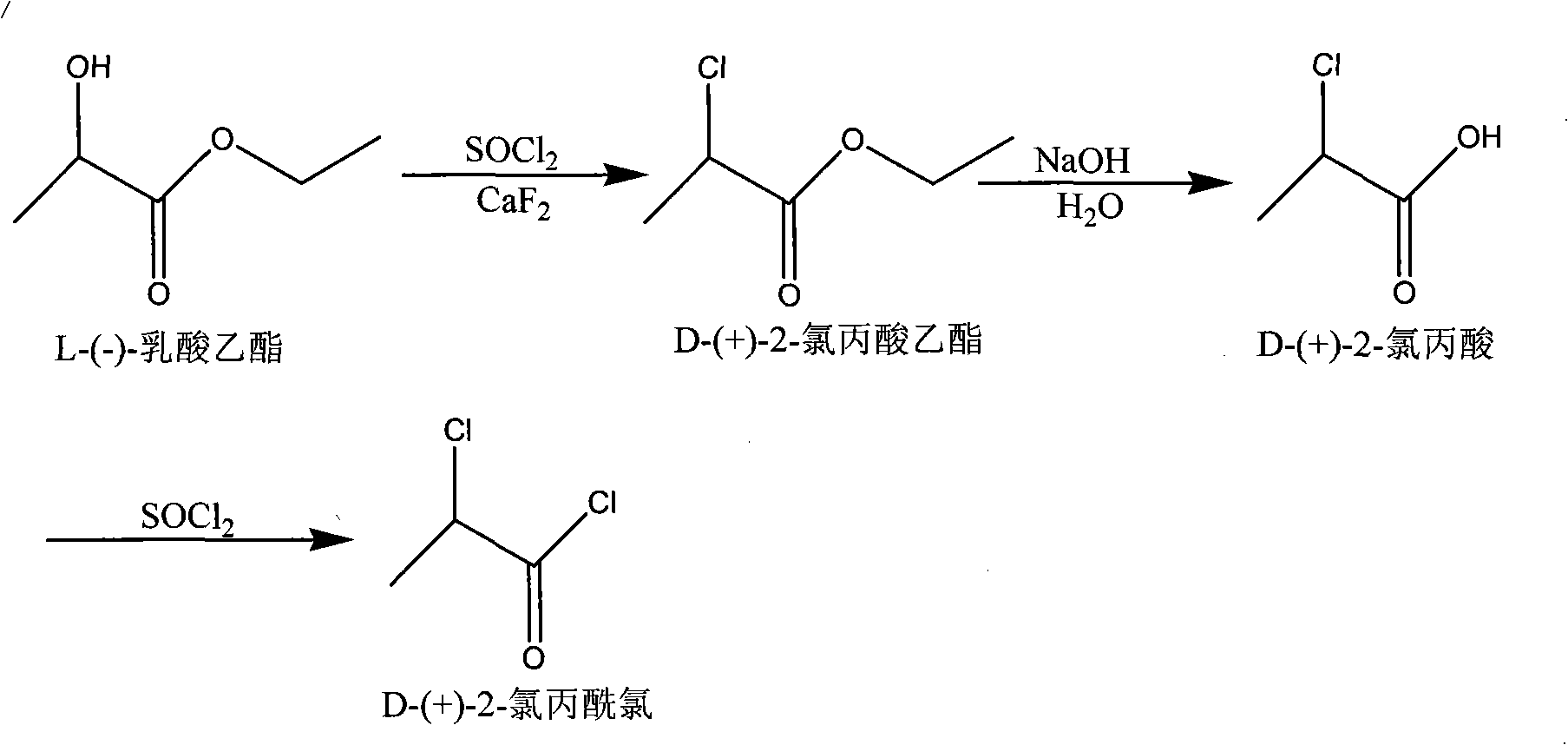
Synthetic method of D-(+)-2-chloro-propanoyl chloride
InactiveCN101284772ASettlement yieldSolve productivityOrganic compound preparationCarboxylic compound preparationPropanoyl chloride2-Chloropropionic acid
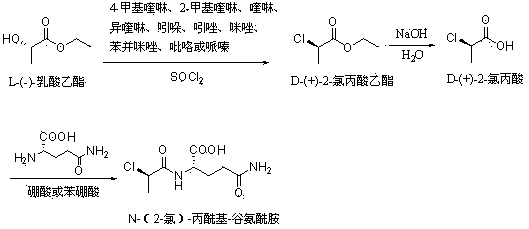
The invention relates to a method for synthesizing D-(+)-2-chloro-Propanoyl chloride, which is used for solving the problems that the product recovery rate of the prior synthetic method is low and the synthetic method is not suitable for industrial production. The method adopts the technical proposal that L-ethyl lactate and thionyl chloride react under the action of calcium fluoride catalyst to generate D-(+)-2-chloropropionic acid ethyl ester, D-(+)-2-chloropropionic acid ethyl ester is hydrolyzed under the action of caustic soda dissolving to generate D-(+)-2-chloropropionic acid, and D-(+)-2-chloropropionic acid reacts with the thionyl chloride to generate D-(+)-2-chloro-Propanoyl chloride. The method has the advantages that the method is novel, the process is simple, the product recovery rate is high, the cost of the catalyst is low, the catalyst is easy to obtain, the environment can not be affected, and the method is suitable for industrial production.
Owner:HEBEI HUACHEN PHARMA +1
Synthesis method of R-(+)-2-(4-hydroxyphenoxy) propionic acid
InactiveCN108314619ARaw materials are easy to obtainThe synthetic route is simpleOrganic compound preparationCarboxylic compound separation/purificationWater bathsIce water
The invention discloses a synthesis method of R-(+)-2-(4-hydroxyphenoxy) propionic acid. The method comprises the following steps: (1), adding hydroquinone into a sodium hydroxide solution containingan organic solvent in batches, and performing reaction under in N2 atmosphere, so as to obtain p-hydroxy sodium phenate turbid liquid; (2), dissolving S-(-)-2-chloropropionic acid in an organic solvent, and slowly adding Na2CO3 solid in an ice-water bath condition to perform reaction, so as to obtain an S-(-)-2-sodium chloropropionic acid solution; (3), slowly dropwise adding the solution in the step (2) into the turbid liquid in the step (1), and performing reduced pressure distillation, dissolution and acidification, extraction and reduced pressure distillation on obtained reaction liquid, so as to obtain white solid, namely the R-(+)-2-(4-hydroxyphenoxy) propionic acid. The synthesis method has the advantages of simple synthesis route, mild reaction condition, high product yield, high product optical purity ee value, high recovery rate of hydroquinone, less produced three wastes and low energy consumption.
Owner:NANJING REDSUN BIOCHEM CO LTD +1
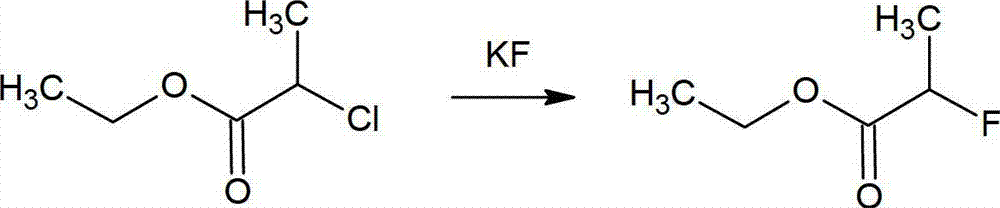
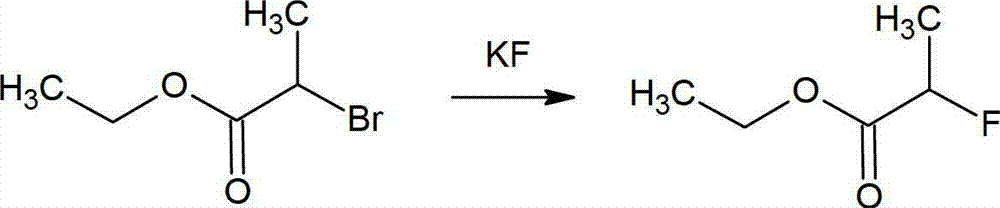
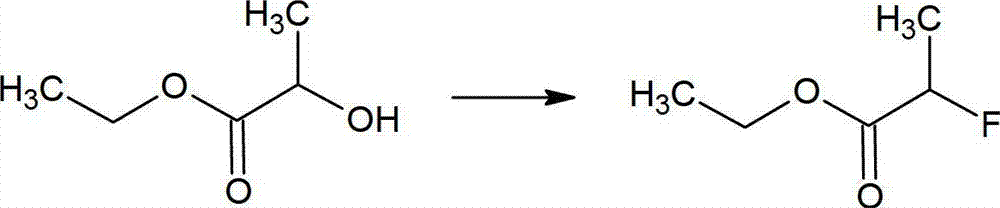
Method for preparing 2-fluoropropionate
ActiveCN103044245AHigh selectivitySimple processOrganic compound preparationCarboxylic acid esters preparationHydrogen fluorideReaction temperature
The invention discloses a method for preparing 2-fluoropropionate, comprising: reacting 2-chloropropionate, hydrogen fluoride and a catalyst according to the quantity ratio of 1:1.2-1.5:0.01-0.02 under 50-200 DEG C for 20-30h, cooling, separating liquid and rectifying after reacting to obtain the 2-fluoropropionate product. The invention has the advantages of high reaction yield, good selectivity of the product, low production cost and little generation amount of three wastes (waste gas,waste water and industrial residue). The reaction yield can reach up to 83.2%, and the highest selectivity of the 2-fluoropropionate is up to 87.5%.
Owner:JUHUA GROUP TECH CENT +1
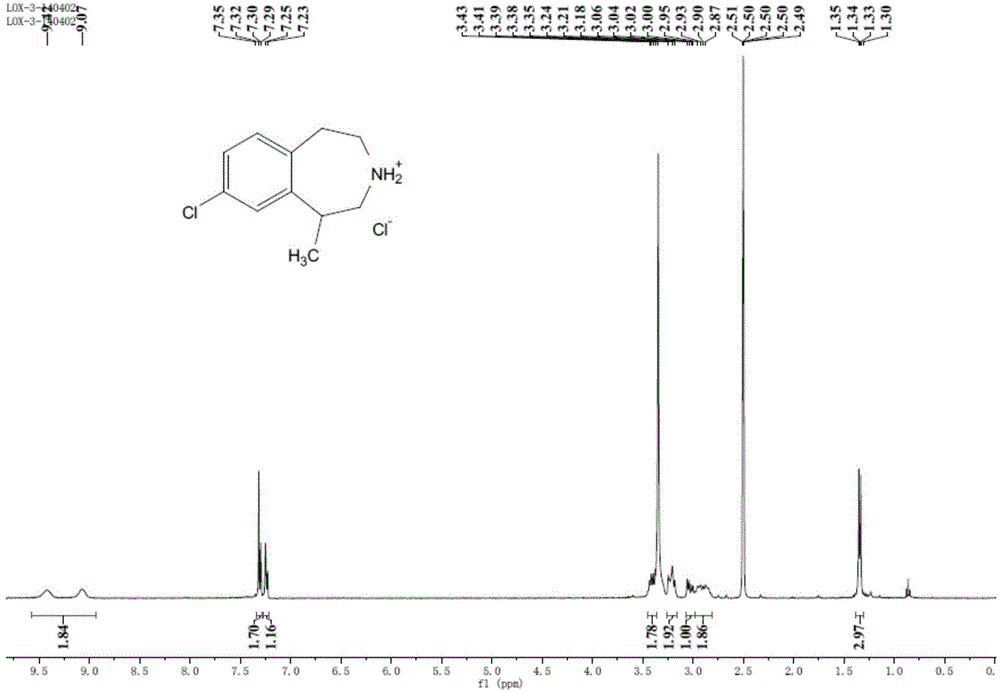
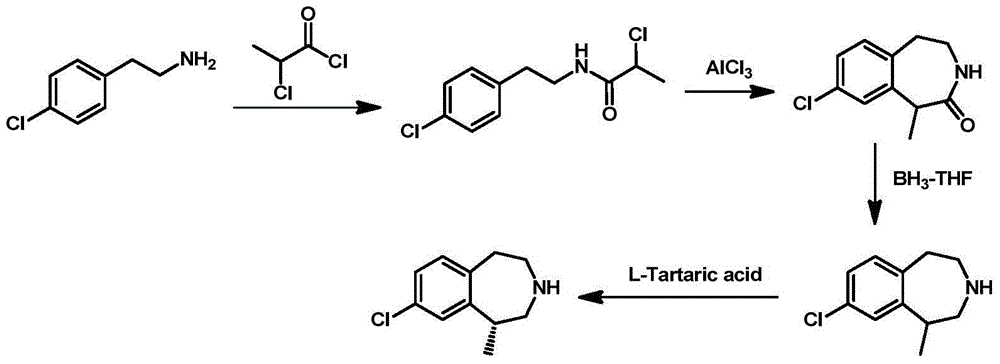
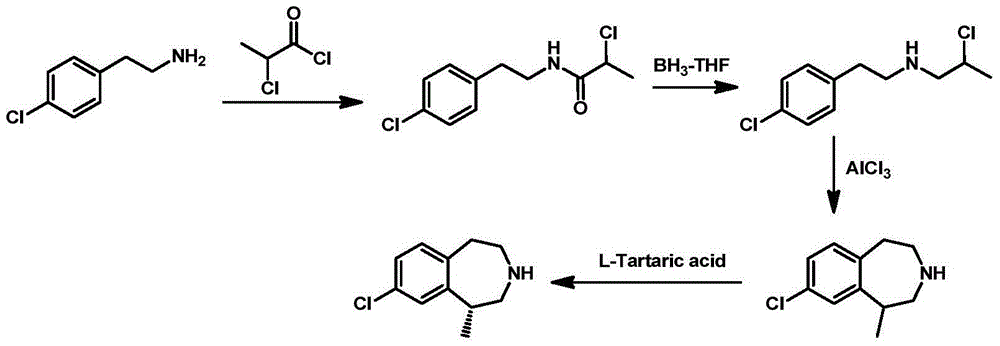
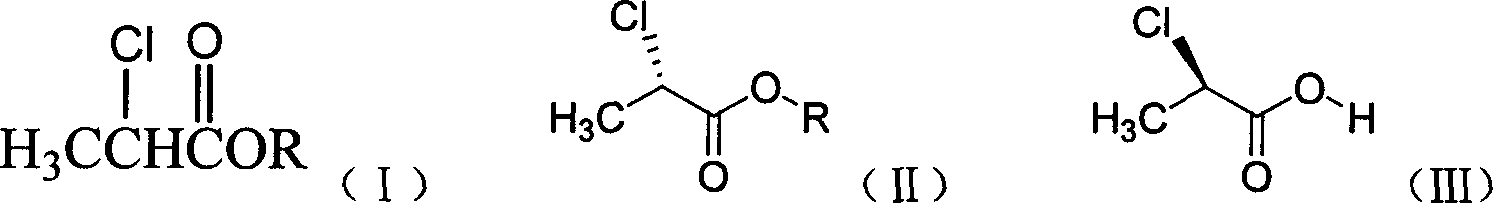
Preparation method of 8-chloro-1-methyl-2,3,4,5- tetrahydro -1H-3-benzoazatropylidene
ActiveCN104130188AWide range of sources and low costEasy to operateOrganic compound preparationCarboxylic acid amides preparationAluminium chlorideCarboxylic acid
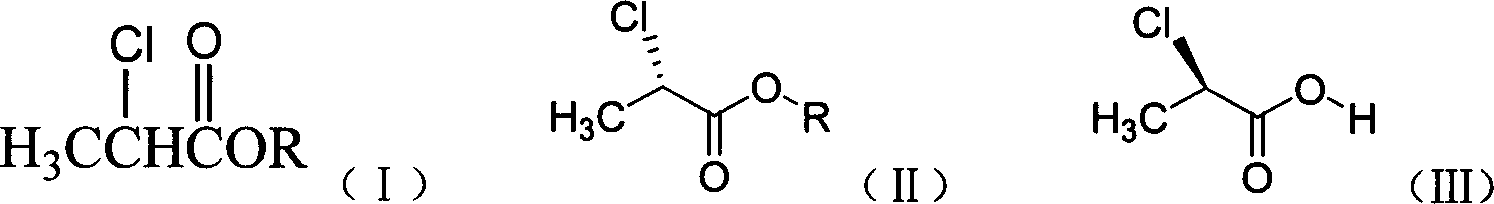
The invention discloses a preparation method of 8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzoazatropylidene. The preparation method comprises the following steps: firstly, enabling a compound with a formula (III) to react with aluminium chloride to obtain a compound with a formula (II); and carrying out reduction reaction on the compound with the formula (II) under the combined action of sodium borohydride and aluminium chloride used in the step i) to obtain a compound in a formula (I). The invention also discloses a method for preparing a compound with a formula (V) or a salt thereof. The method comprises the following steps: with chlorobenzyl cyanide and 2-chloropropionic acid as raw materials, reacting under the existence of a carboxylic acid activation reagent to generate a compound in a formula (IV); and carrying out reduction reaction on the compound with the formula (IV) under the action of a reducing agent to obtain the compound with the formula (V). Compared with the prior art, the method disclosed by the invention has the advantages of low cost, convenience for operation, safety of production, high yield and the like.
Owner:NANJING UNIV OF TECH
Synthesis method of high-purity D-2-chloropropionyl chloride
ActiveCN103408416ALower requirementOperational securityOrganic compound preparationCarboxylic compound preparationSynthesis methodsMethyl lactate
The invention provides a synthesis method of high-purity D-2-chloropropionyl chloride. A process path of chlorination, hydrolysis and chloroacylation of L-methyl lactate is adopted. Specifically, the synthesis method sequentially comprises the following steps: (1) the synthesis of D-methyl-2-chloropropionate: dropwise adding thionyl chloride into the mixed solution of the L-methyl lactate and pyridine, carrying out vacuum stirring, increasing the temperature to 55-90 DEG C, reacting for 1-5h, reducing the temperature to 25-35 DEG C, exhausting air, washing and separating, wherein the L-methyl lactate and the thionyl chloride are used as raw materials and the pyridine is used as a catalyst; (2) the synthesis of D-2-chloropropionic acid: hydrolyzing methyl-2-chloropropionate under the action of a sodium hydroxide solution, and carrying out chloroform extraction; and (3) the synthesis of D-2-chloropropionyl chloride: dropwise adding the thionyl chloride into the mixed solution of D-2-chloropropionic acid and the catalyst, increasing the temperature to 70-80 DEG C, reacting for 3-5 hours, and carrying out vacuum rectification to obtain a target product. The process path has the advantages that raw materials are easily available; synthesis steps are simple, safe and reliable; the waste gas generated in the production can be easily disposed; therefore, the process path is suitable for industrial production.
Owner:SHANDONG JINCHENG PHARMACEUTICAL GROUP CO LTD
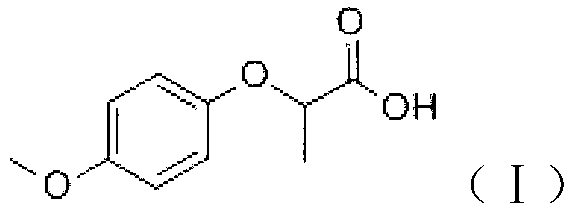
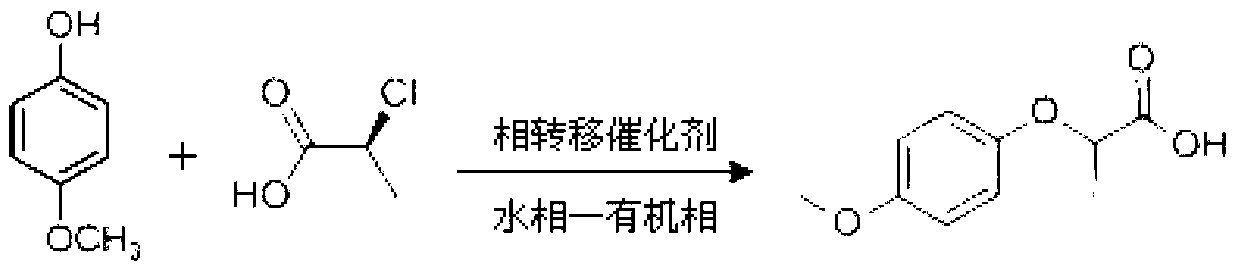
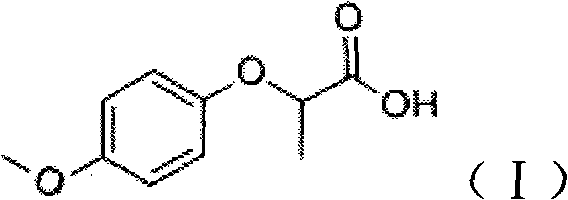
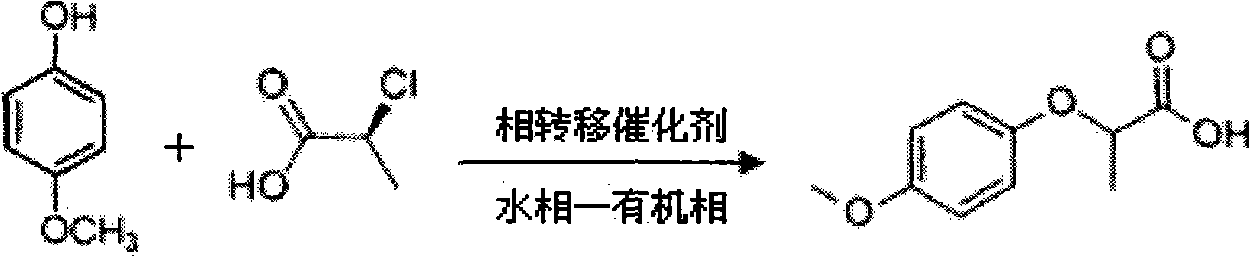
Industrial method for synthesizing 2-(4-methoxyphenoxy)-propionic acid through phase transfer
ActiveCN102010326AImprove conversion efficiencyGuaranteed conversion efficiencyOrganic compound preparationCarboxylic compound preparation4-MethoxyphenolHydroxybenzoate Ethers
The invention discloses an industrial method for synthesizing 2-(4-methoxyphenoxy)-propionic acid through phase transfer, which comprises the following steps of: stirring and mixing 4-Methoxyphenol, sodium hydroxide, a phase transfer catalyst and water, and reacting at normal temperature and normal pressure for 10 to 20 minutes to obtain mixed feed liquid; adding 2-chloro-propanoic acid and an organic phase into the mixed feed liquid, and reacting for 0.5 to 1.5 hours to obtain a product of 2-(4-methoxyphenoxy)-propionic acid; regulating the reaction liquid subjected to reaction to be acid byusing HCl or sulfuric acid solution, and standing to separate a water phase and the organic phase; and evaporating organic phase separation liquid to remove a solvent, and performing acid-base recrystallization to obtain the 2-(4-methoxyphenoxy)-propionic acid. When the reaction is finished, the yield of the 2-(4-methoxyphenoxy)-propionic acid is over 90 percent, the reaction temperature is reduced to 40-60 DEG C, the reaction time is only 0.5 to 1.5 hours, and the content of 2-(4-methoxyphenoxy)-propionic acid in the final product is 99.5 percent.
Owner:SOUTH CHINA UNIV OF TECH
Synthesizing method of L-2-sodium chloropropionate
InactiveCN105732358AHigh yieldEmission reductionOrganic compound preparationCarboxylic acid salt preparationFiltration2-Chloropropionic acid
The invention discloses a synthesizing method of L-2-sodium chloropropionate.The synthesizing method comprises the steps that hydrochloric acid is directly sucked or pumped into a reaction kettle, and L-2-aminopropionic acid solids are put into the reaction kettle; the temperature is lowered to 0 DEG C or below through an ice bath, prepared sodium nitrite is added in batches, stirring is stopped after reacting is finished, and standing under heat preservation is performed for 2-3 hours; suction filtration is performed, L-2-chloropropionic acid filtrate obtained through suction filtration is contained in a separating funnel oscillator to be extracted, obtained extracting solutions are merged after layering under standing is performed, alkali liquid is added into the merged extracting solution for a neutralization reaction, layering under standing is performed, an obtained oil layer is dichloromethane solvent, and a water layer is an aqueous solution of L-2-sodium chloropropionate.According to the synthesizing method of L-2-sodium chloropropionate, the alkali liquid is directly added into the dichloromethane extracting solution of L-2-chloropropionic acid for neutralization, distilling is replaced with layering, the whole dichloromethane distilling and recycling process is omitted, and therefore not only is the efficiency greatly improved, but also energy consumption is greatly reduced.
Owner:福建仁宏医药化工有限公司
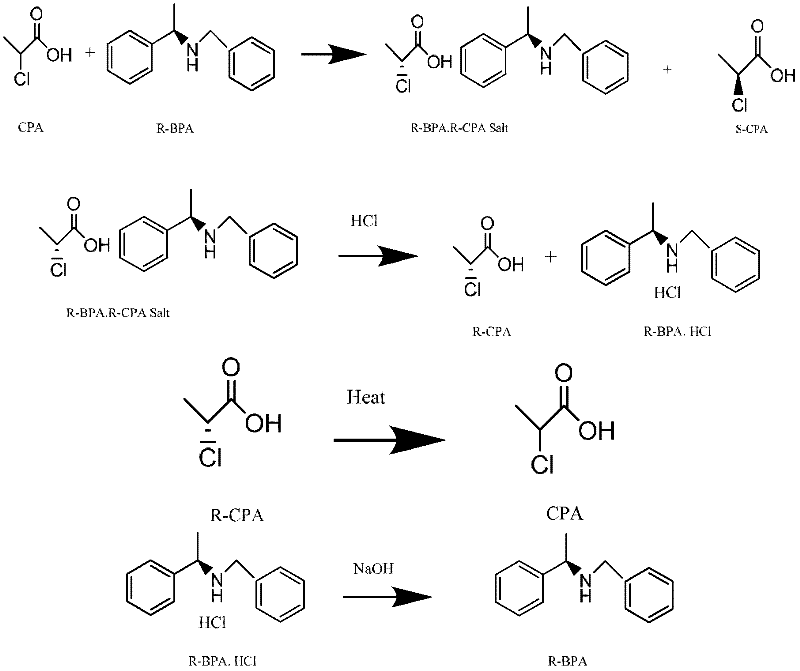
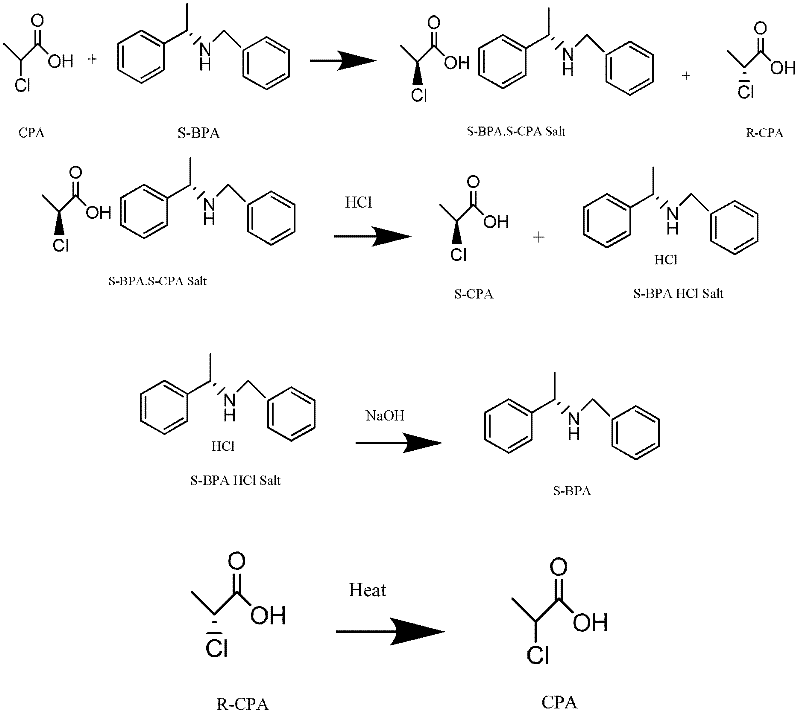
Method for preparing chiral (S)-2-propionic acid
InactiveCN102344355ASolve pollutionNo pollution in the processChemical recyclingCarboxylic compound separation/purificationPropanoic acidAlcohol
The invention relates to a method for preparing (S)-2-propionic acid. The method comprises the following step of: splitting racemized 2-propionic acid in an alcohol solvent by using chiral N-benzyl-phenylethylamine BPA (Bisphenol A) to obtain (S)-2-propionic acid. Due to the adoption of the preparation method disclosed by the invention, the problem of environmental pollution caused by an L-alanine method can be fully solved, all splitting reagents and (R)-2-propionic acid obtained by splitting are fully recycled after racemization, waste is not produced basically, environmental pollution is avoided, the aims of saving energy, protecting the environment, reducing emission and lowering emission are fulfilled, the production cost is greatly lowered, the market competitiveness of the product is improved, and good industrial prospect is achieved. The (S)-2-propionic acid obtained with the method has high chemical purity and high chiral purity.
Owner:上海科利生物医药有限公司
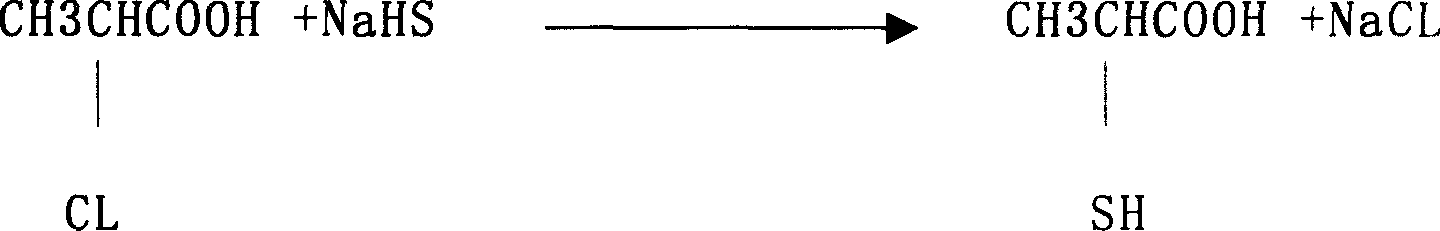
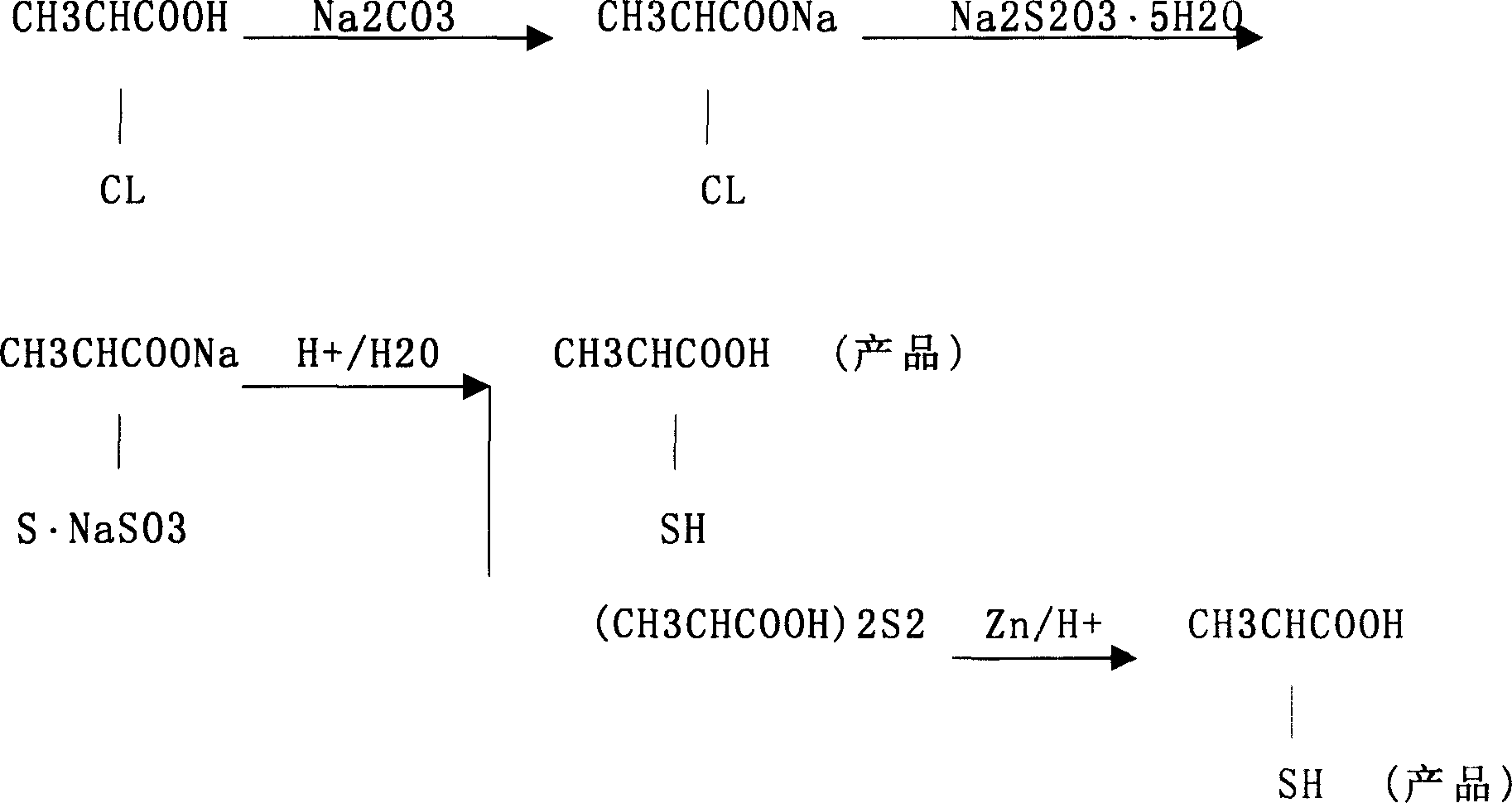
Prepn process of 2-mercapto propanoic acid
The preparation process of 2-mercapto propanoic acid includes the following steps: mixing water solution of 2-chloropropionic acid and water solution of sodium carbonate to obtain sodium 2-chloropropionate in the reacted liquid; adding sodium thiosulfate into the reacted liquid to form mixture solution of sodium 2-thiopropionate, sodium 2-mercapto propanoic acid, sodium 2-mercapto propionate, sodium 2-thiodipropionate and 2-thiodipropionic acid; adding acid into the mixture solution to acidify to obtain mixture solution with 2-thiodipropionic acid and other acids; and finally adding zinc powder into the mixture solution to reduce 2-thiodipropionic acid into 2-mercapto propanoic acid in solution. The process has cheap material, low production cost, complete utilization of the material and high yield of 2-mercapto propanoic acid.
Owner:顾建荣
Method of preparing (S)-(-)-2-chloropropionate and (R)-(+)-2-chloro propionic acid
InactiveCN1868997AThe reaction process is simpleHigh selectivityOrganic compound preparationCarboxylic acid esters preparationPropanoic acid2-Chloropropionic acid
A process for preparing (S)-(-)-2-chloropropionate and (R)-(+)-2-chloropropionic acid includes such steps as selective hydrolytic reaction of dl-2-chloropropionate in reaction medium under existence of pig steapsase, and separating two products from the resultant liquid.
Owner:ZHEJIANG UNIV OF TECH
Process for preparing high-optical-purity R-(+)-2-chloropropionic acid through ester exchange method
ActiveCN104370691ANormal catalytic reactionReduce oxidationOxygen-containing compound preparationOrganic compound preparationStrong acids2-Chloropropionic acid
The invention discloses a process for preparing high-optical-purity R-(+)-2-chloropropionic acid through an ester exchange method. The process comprises the following steps: placing ethyl chloropropionate in a reactor, sequentially adding formic acid with the mass fraction of 94% and strong-acid cation exchange resin under stirring, slowly heating to 54DEG C, extracting ethyl formate, continuously heating, and recovering formic acid; and forming a negative pressure after the recovery of formic acid, and extracting R-(+)-2-chloropropionic acid. The process has the advantages of simple technology, no strong acidic tails, environmental protection and short production period, and the optical purity and the yield of the prepared R-(+)-2-chloropropionic acid are not smaller than 99% and not smaller than 90% respectively.
Owner:HUBEI BIOCHEM PHARMA TECH
Method for synthesizing (R)-2-chloropropionic acid by solid acid catalysis and trans-esterification reaction based on UIO-66
ActiveCN106748713AImprove conversion rateRapid responseOrganic-compounds/hydrides/coordination-complexes catalystsPreparation from carboxylic acid esters/lactonesTrans esterificationSolid acid
The invention provides a method for synthesizing (R)-2-chloropropionic acid by a solid acid catalysis and trans-esterification reaction based on UIO-66. The method comprises the following steps: (1) preparation of a solid acid catalyst SO4<2> / UIO-66; and (2) preparation of (R)-2-chloropropionic acid. According to the method, UIO-66 is adopted as a solid acid catalyst of a substrate, the catalyst has strong acidity, so that the catalyst can effectively catalyze the trans-esterification reaction for synthesizing (R)-2-chloropropionic acid, the transformation rate is higher than 98%, side effects are less, and the catalyst can be used repeatedly, and therefore, the generation of tailings is effectively avoided, the production cost is lowered, and environmental protection is facilitated.
Owner:HUBEI BIOCHEM PHARMA TECH +1
2-fluoropropionate synthetizing method
ActiveCN105037150AMild reaction conditionsHigh yieldOrganic compound preparationCarboxylic acid esters preparationPotassium fluoride2-Chloropropionic acid
The invention discloses a 2-fluoropropionate synthetizing method. The method comprise the steps of 1, adding ester to a four-mouth flask provided with a condenser pipe, wherein the general formula of the ester is CH3CHXCOOR, and R represents alkyl; 2, adding an addition agent, a catalyst and solvent to the four-mouth flask, and then adding a fluorinated reagent and conducting stirring to obtain reaction liquid; 3, heating the reaction liquid to a set temperature, wherein reaction time is 3-16 h; 4, conducting rectification after reaction ends to obtain a 2-fluoropropionate target product. According to the method, cheap 2-CH3CHClCOOR is used as the raw material, and fluorination is conducted with potassium fluoride in the presence of the addition agent to obtain the 2-fluoropropionate. The method has the advantages that the reaction condition is mild, the yield is high, and cost is low. Due to the fact that the addition agent is added, generation of high-boiling by-products can be restrained, and then reaction selectivity is improved.
Owner:JUHUA GRP
Synthesis method of N-(2-chloride)-propionyl-glutamine
InactiveCN103467334AThe process is simple and feasibleHigh yieldOrganic compound preparationCarboxylic acid amides preparationSynthesis methods2-Chloropropionic acid
The invention discloses a synthesis method of N-(2-chloride)-propionyl-glutamine. The synthesis method comprises the steps of reacting L-ethyl lactate with thionyl chloride under the presence of catalyst, distilling reaction liquor into alkaline liquor for hydrolyzing to obtain 2-chloropropionic acid; reacting the 2-chloropropionic acid with L-glutamine to obtain N-(2-chloride)-propionyl-glutamine under the presence of the catalyst, and other steps. The synthetic route is simple, the synthesis method is novel, simple and convenient in technique, and high in product yield and purity, the catalyst is low-cost and easily available, the environment is not influenced, and N-(2-chloride)-propionyl-glutamine is suitable for industrial production.
Owner:CHONGQING TECH & BUSINESS UNIV
[ethyl (R)-(+)-beta-(1- methylimidazolyl)-propionate]X chiral ionic liquid and synthesis method thereof
The invention discloses a [ethyl (R)-(+)-beta-(1-methylimidazole)-propionate]X chiral ionic liquid and a synthesis method which is used for solving the technical problem that imidazole type chiral ionic liquid prepared by the existing preparation method has high liquid melting point and low yield. The technical scheme is as follows: (S)-(-)-2-chloropropionic acid and (S)-(-)-2-bromopropionic acid are taken as raw materials and undergo esterification reaction with absolute ethyl alcohol to generate stable (S)-(-)-2-chloropropionic acid ethyl ester or (S)-(-)-2-bromopropionic acid ethyl ester; the (S)-(-)-2-chloropropionic acid ethyl ester or (S)-(-)-2-bromopropionic acid ethyl ester undergoes alkylation reaction with N- methylimidazole to generate chlorinated ethyl (R)-(+)-beta-(1-methylimidazole)-propionate or brominated ethyl (R)-(+)-beta-(1- methylimidazole)-propionate chiral ionic liquid; the chlorinated ethyl (R)-(+)-beta-(1- methylimidazole)- ropionate or brominated ethyl (R)-(+)-beta-(1- methylimidazole)-propionat chiral ionic liquid is used as an intermediate to undergo the anion exchange reaction with salts containing anions such as BF4<->, PF6<->, SCN<->, CH3COO<-> so as to obtain the [ethyl (R)-(+)-beta-(1- methylimidazole)-propionate] X chiral ionic liquid. The melting point of the [ethyl (R)-(+)-beta-(1- methylimidazole)-propionate] X chiral ionic liquid is reduced to minus 40 to minus 36 DEG C from 5 to 16 DEG C of the melting point of the background art; the yield of the [ethyl (R)-(+)-beta-(1- methylimidazole)-propionate] X chiral ionic liquid is increased to 52.8 to 60.5% from 20 to 35% of yield of the background art.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
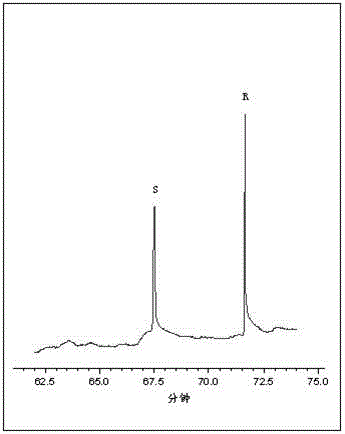
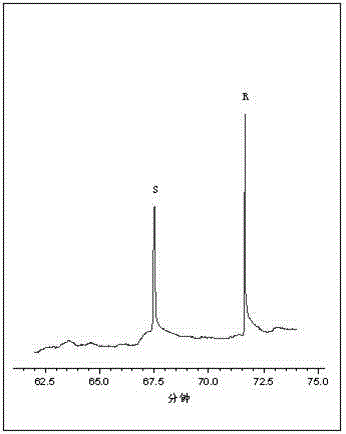
Method for resolution of racemization 2-chloropropionic acid by adopting capillary electrophoresis separation-diode array detection technique
InactiveCN104965018AChiral separation achievedEasy to operateMaterial analysis by electric/magnetic meansColor/spectral properties measurements2-Chloropropionic acidPeak area
The invention discloses a method for resolution of racemization 2-chloropropionic acid by adopting a capillary electrophoresis separation-diode array detection technique. The method comprises the steps of: adding a chiral resolution agent, an organic solvent and a cation surfactant into a prepared buffer solution respectively and performing ultrasonic mixing to obtain a capillary electrophoresis operation buffer solution; using the buffer solution as an electrophoresis medium for resolution to obtain R-2-chloropropionic acid and S-2-chloropropionic acid monomers; calculating an enantiomer excessive value according to a chromatogram peak area; monitoring product quality according to the value. The method can achieve base line separation of the R-2-chloropropionic acid and S-2-chloropropionic acid monomers, and has the characteristics of good separation degree, low cost and simple and convenient operation.
Owner:HUBEI BIOCHEM PHARMA TECH +1
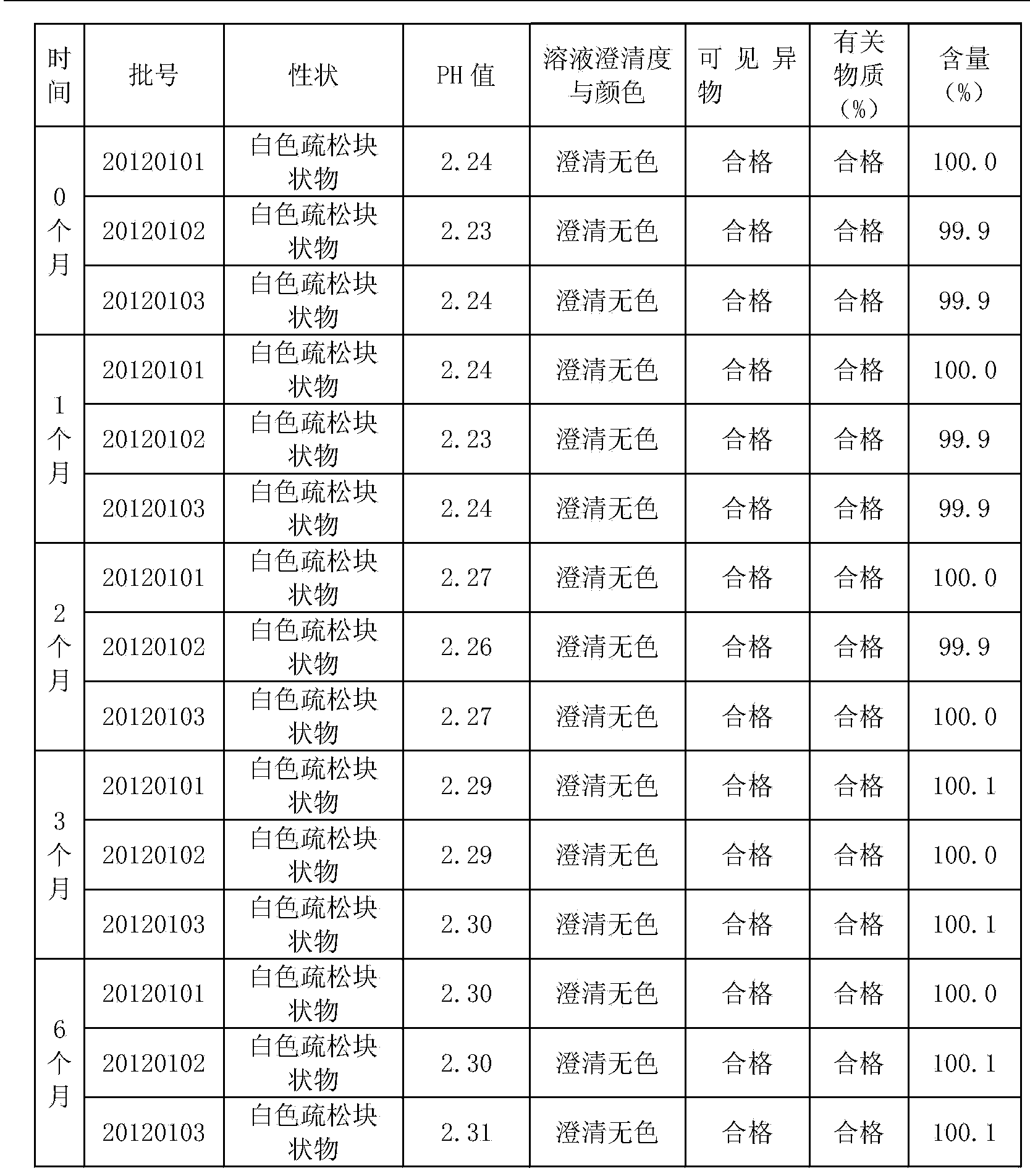
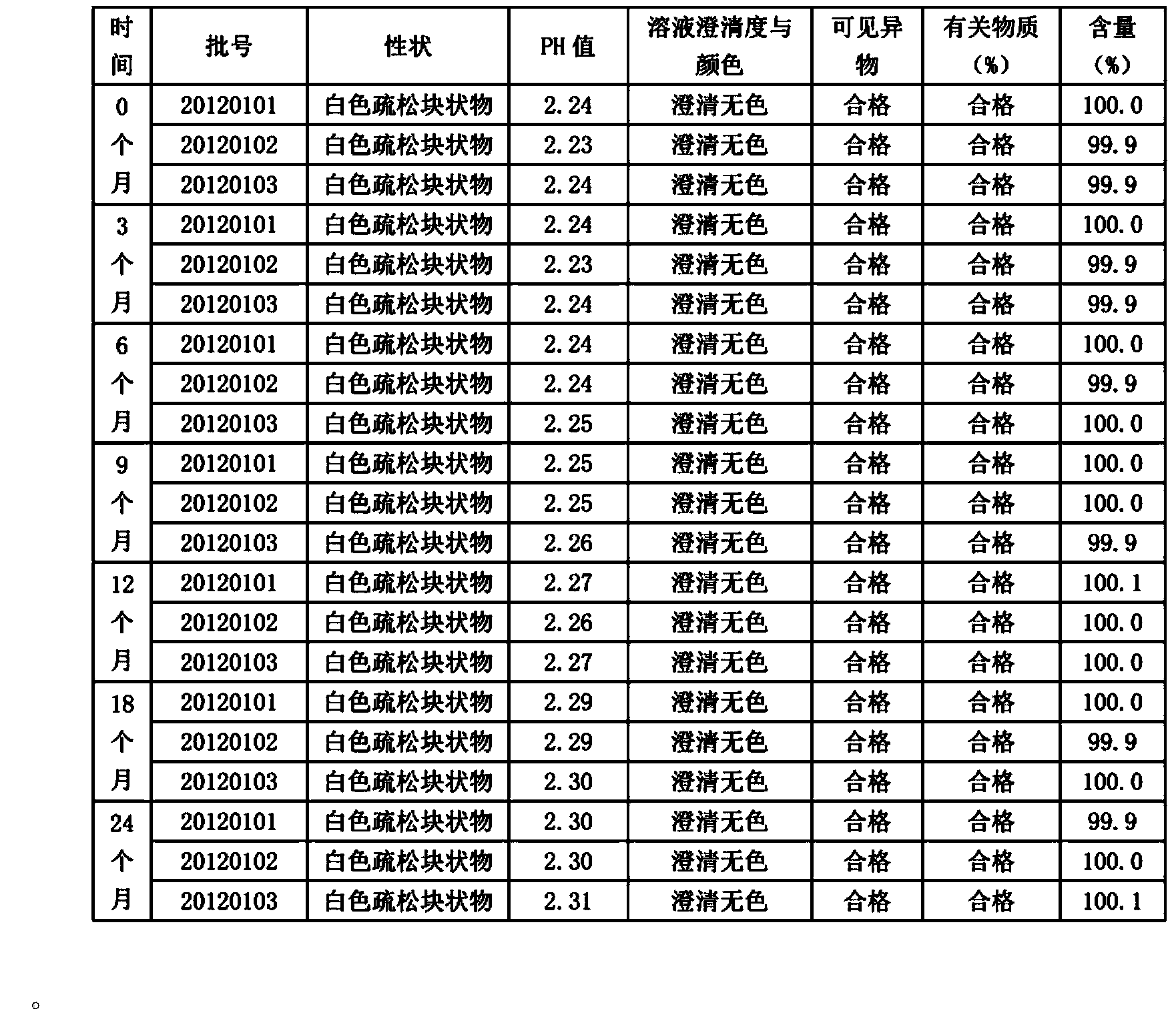
Special ultrafine tiopronin powder lyophilized preparation and preparation method thereof
InactiveCN104163781AHigh clarityImprove stabilityOrganic active ingredientsPowder deliverySolubility2-Chloropropionic acid
The invention discloses a special ultrafine tiopronin powder lyophilized preparation and a preparation method thereof. The preparation method comprises the following steps: reacting 2-chloropropionic acid with phosphorus trichloride to generate 2-chloropropionyl chloride, reacting 2-chloropropionyl chloride with glycine to generate 2-chloropropionyl glycine, reacting 2-chloropropionyl glycine with sodium disulfide, reducing by zinc powder to obtain crude tiopronin, purifying, carrying out air jet ultrafine crushing, and lyophilizing to prepare the special ultrafine tiopronin powder lyophilized preparation. The special ultrafine tiopronin powder lyophilized preparation has the advantages of good clarity, high stability, high purity, few impurities, small particle size, large specific surface area, good solubility, small toxic side effects, difficult allergy and the like.
Owner:杭州长典老一元健康管理有限公司
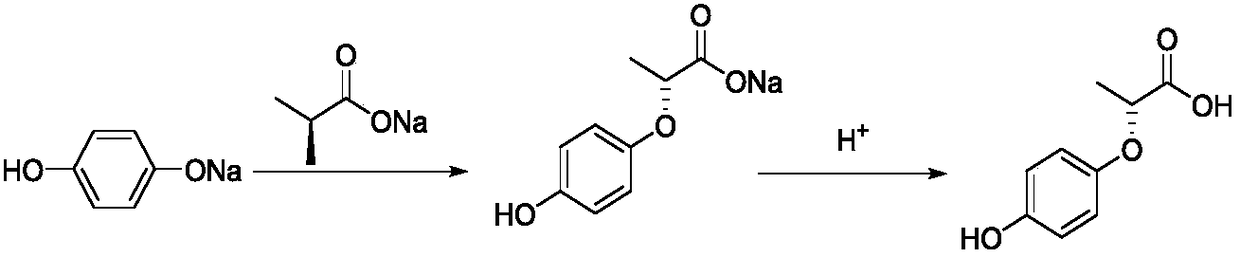
Ethyl(2R)-2-[4-(4-cyano 2-fluorophenoxy)-phenoxy]propionic ester, and preparation and applications thereof
InactiveCN107183028AShort yieldSimple processBiocideCarboxylic acid nitrile preparationBristlegrassesPropanoic acid
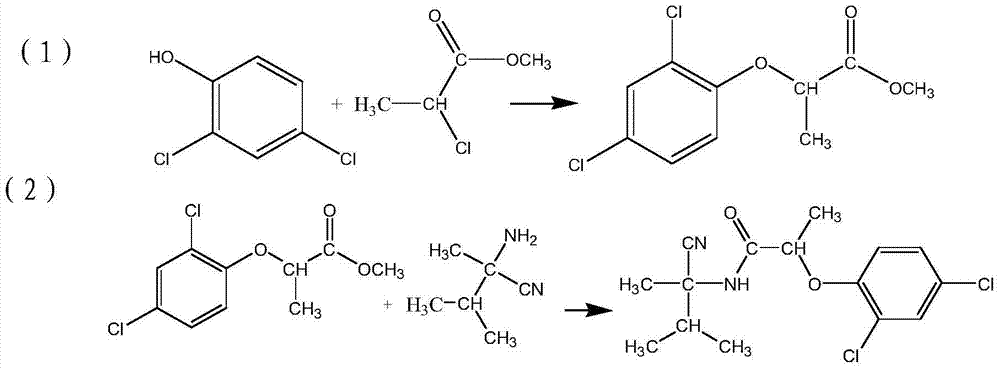
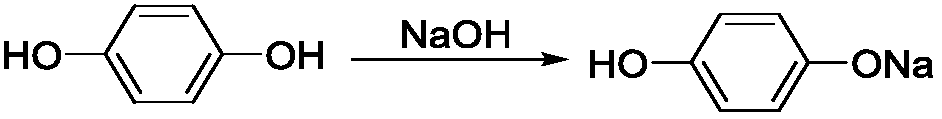
The invention provides a weeding compound used for killing and preventing gramineous weeds in paddy fields. The weeding compound is ethyl(2R)-2-[4-(4-cyano 2-fluorophenoxy)-phenoxy]propionic ester. A preparation method comprises following steps: hydroquinone and (S)-(-)-2-chloropropionic acid are subjected to Williamson etherification reaction at alkaline conditions so as to obtain (R)2-[4-hydroxy-phenoxy]propionic acid; reaction with a strong base is carried out so as to obtain (R)2-[4-(4-cyano 2-fluorophenoxy)-phenoxy]propionic acid; and reaction with a strong base is carried out so as to obtain ethyl(2R)-2-[4-(4-cyano 2-fluorophenoxy)-phenoxy]propionic ester. The preparation method is simple; reaction is mild; no high temperature high pressure condition is needed; reaction time is relatively short; yield is high; the weeding compound can be used for killing Euphorbia lathyris with high efficiency, and can also be used for killing crab grass, paspalum distichum, green bristlegrass, eleusine indica, and alopecurus aequalis, and possesses excellent killing effect on barnyard grass with drug resistance; and the using amount is less than that of cyhalofop-butyl.
Owner:燕化永乐(乐亭)生物科技有限公司
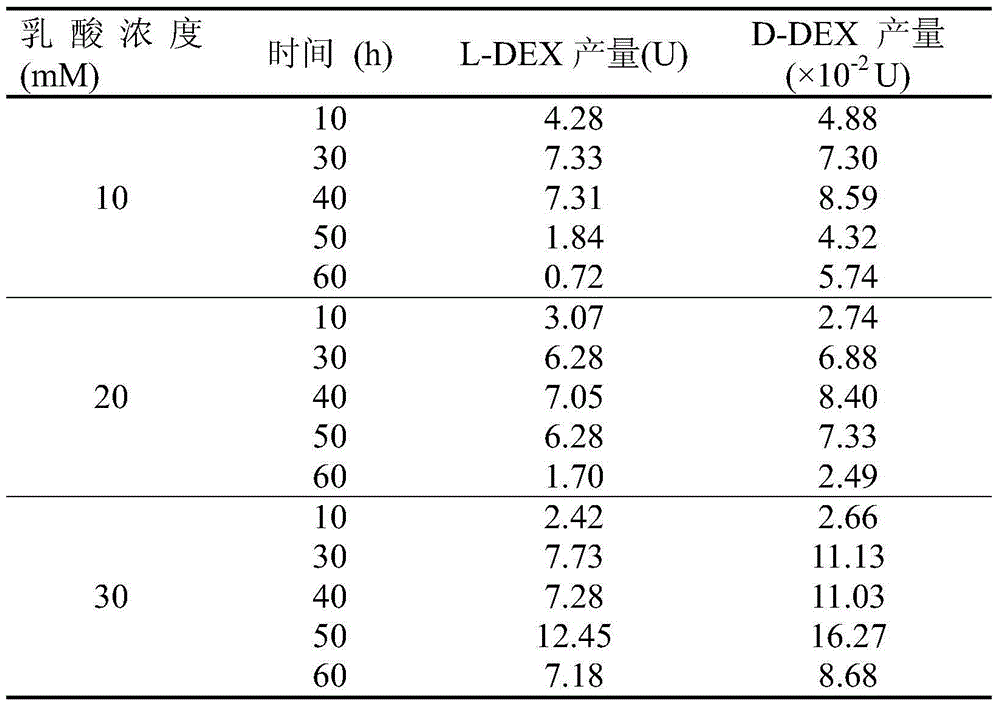
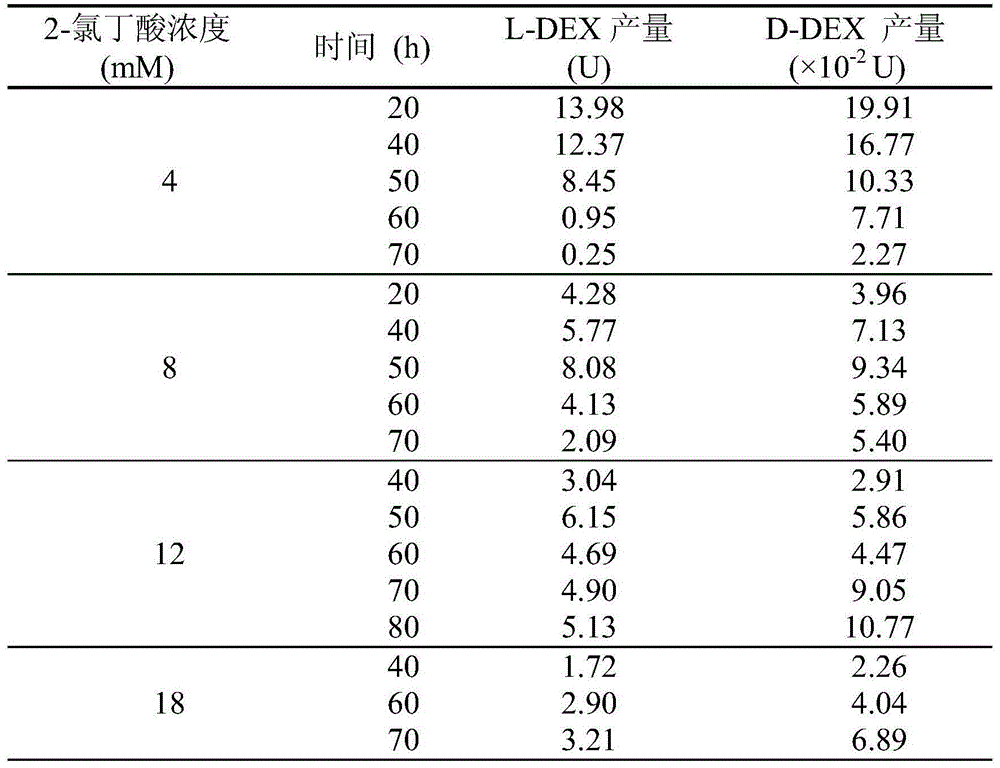
Method for selectively increasing haloacid dehalogenase yield
InactiveCN105483095AIncrease productionStable outputHydrolasesMicroorganism based processesHydroxybutyric acid3-chloropropionic acid
The invention discloses a method for selectively increasing haloacid dehalogenase yield. Pseudomonas strains DEH138 are adopted according to a mechanism that inducers induce microorganisms to generate induced enzymes, and a proper carbon source is selected and optimized to specifically increase L-2-haloacid dehalogenase yield. To be more specific, inducer halogenated compounds (chloroacetic acid, iodoacetic acid, 2-propanoate, 2-chloropropionic acid, 2-bromopropionate, 3-chloropropionic acid, 2-chlorobutyric acid, 2-bromobutyric acid, 2-chloride propionamide and 2-bromide propionamide) are added in a logarithmic phase or synchronously in mineral basal media containing auxiliary carbon sources (glycolic acid, lactic acid, hydroxybutyric acid or glucose) respectively, so that induced expression of L-2-haloacid dehalogenase can be promoted; meanwhile, quantity of D-2-haloacid dehalogenases different in chiral selectivity is kept stable, and the L-2-haloacid dehalogenase yield is finally selectively increased. The method is a novel production mode for acquiring dehalogenases with chiral selectivity.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Synthesis method of(R)-2-[4-(4-cyano-2-fluorophenoxy)phenoxy] propionic acid
ActiveCN110563606AAtom utilization is highHigh reaction yieldCarboxylic acid nitrile preparationOrganic compound preparationP-ChlorophenolPropanoic acid
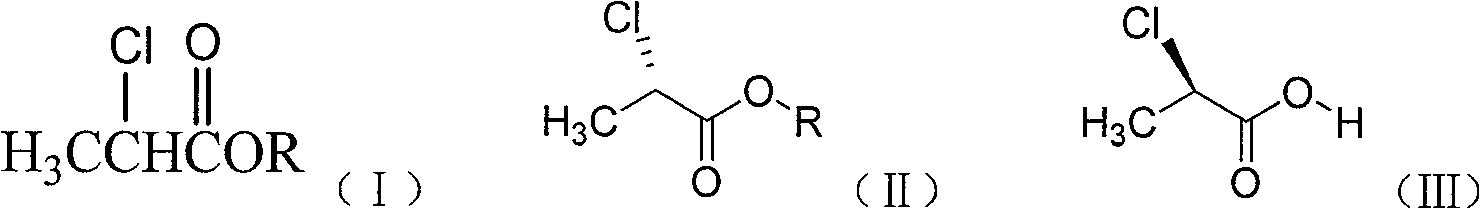
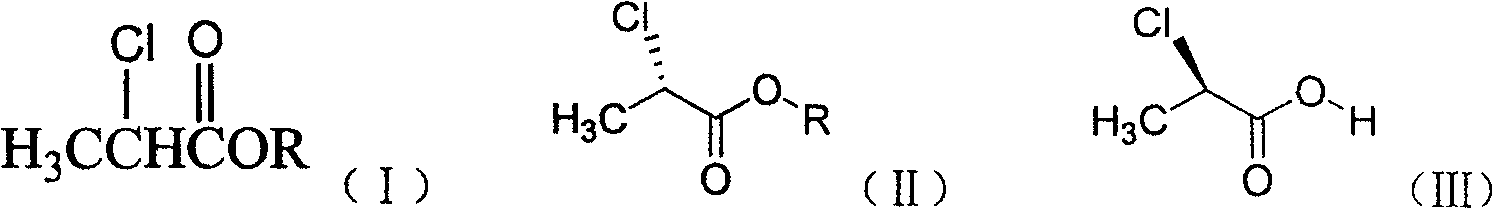
The invention discloses a method for synthesizing cyhalofop butyl intermediate(R)-2-[4-(4-cyano-2-fluorophenoxy)phenoxy] propionic acid.(S)-2-chloropropionic acid methyl ester and p-chlorophenol are used as raw materials, an etherification reaction is performed under an alkaline condition to generate(R)-2-(4-hydroxyphenoxy)propionic acid, and the etherification product and 3-fluoro-4-hydroxybenzonitrile generate the(R)-2-[4-(4-cyano-2-fluorophenoxy)phenoxy] propionic acid under the action of an acid-binding agent. According to the method, the problems of serious excessive hydroquinone servingas a raw material, massive use of an inorganic base and generation of dietherification byproducts in synthesis are avoided. The method is high in atom utilization rate, high in reaction yield and lowin production cost.
Owner:湖南速博生物技术有限公司
Method for synthesizing fenoxanil
ActiveCN104496847AReduce typesLow costCarboxylic acid nitrile preparationOrganic compound preparationSodium bicarbonateDistillation
The invention relates to a novel method for synthesizing fenoxanil serving as a systemic fungicide. The novel method for synthesizing fenoxanil serving as the systemic fungicide comprises the following steps: adding 2,4-dichlorophenol, sodium hydroxide and 2-methyl chloropropionate into a methyl benzene solvent, controlling reaction temperature at 30-70 DEG C, washing methyl benzene phase by using water after the reaction is ended, and carrying out reduced pressure distillation on a solvent of the washed methyl benzene phase to obtain dichlorprop methyl ester; and adding the obtained dichlorprop methyl ester into a methyl benzene solution with dissolved 2-amino-2,3-dimethyl butyronitrile and sodium bicarbonate, reacting by controlling the reaction temperature at 0-30 DEG C, washing the methyl benzene phase by using water after the reaction is ended, carrying out reduced pressure distillation on a solvent of the washed methyl benzene phase, recrystallizing by using a mixed solvent of water and ethyl alcohol, reducing the temperature to the room temperature, and filtering to obtain the fenoxanil. According to the synthesis method, 2-chloropropionic acid is replaced by 2-methyl chloropropionate; dipropionyl chloride is replaced by the generated dichlorprop methyl ester; one-step acylation reaction is eliminated; the process route is simplified; the reaction period is shortened; the method has great development in the industrial production.
Owner:JINGBO AGROCHEM TECH CO LTD
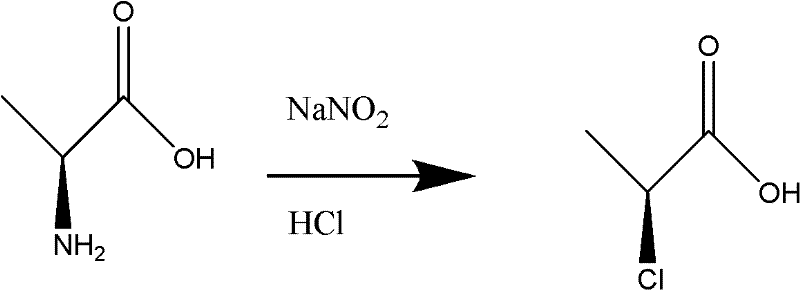
Synthetic method of high-purity and high-yield L-2-chloropropionic acid
InactiveCN107879925AImprove conversion rateHigh yieldOrganic compound preparationCarboxylic compound preparation2-Chloropropionic acidEthyl Chloride
The invention discloses a synthetic method of L-2-chloropropionic acid. The synthetic method comprises the steps of firstly dissolving L-alanine into hydrochloric acid, introducing nitrosyl chloride and hydrogen chloride gas for reaction, after the raw materials are converted, filtering, continuing to stir for 1-2 hours, and an alkaline reagent for neutralization, extracting by virtue of an organic solvent, combining organic phases, drying by virtue of anhydrous calcium chloride, filtering, and carrying out rectification, so as to obtain a product, namely L-2-chloropropionic acid. The method has the advantages that the operation is simple, and the emission of wastewater is reduced; and by taking nitrosyl chloride with relatively high activity as a diazotization regent, aliphatic amino canbe rapidly converted into chlorine, so that the raw material conversation rate and the yield are greatly increased, the yield can reach 90% or above, an original configuration is not reversed in the reaction process, and the optical purity ee value can reach 99.1%.
Owner:NANJING REDSUN BIOCHEM CO LTD +1
Method for synthesizing intermediate of agricultural bactericide fenoxanil
InactiveCN101624341AEmission reductionIncreased condensation yieldOrganic compound preparationCarboxylic compound preparationPropanoic acidSynthesis methods
The invention discloses a method for synthesizing the intermediate of agricultural bactericide fenoxanil and relates to a process for synthesizing an agricultural intermediate. The method comprises the following steps: putting 2,4-dichlorophenol, 2-chloropropionic acid, potassium hydroxide and surfactant in a dimethyl sulfoxide solvent, keeping a reaction temperature between 20 and 80 DEG C, keeping the temperature after the reaction is finished, neutralizing the reaction solution with sulfuric acid, filtering the neutralized reaction solution and distilling filtrate at a reduced pressure to remove the solvent; and taking a solid substance, adding normal hexane into the solid substance, cooling the mixture to room temperature, and filtering the mixture to obtain 2-(2,4-dichlorophenoxy) propionic acid. Using a solvent in place of water as a medium, the synthesis method of the invention improves condensation yield to 90 percent, reduces the discharge of water containing phenol during reaction and reduces production cost by recycling the solvent.
Owner:JIANGSU CHANGQING AGROCHEMICAL CO LTD
Continuous synthesis method of diethyl methylmalonate
InactiveCN109020810AImprove responseHigh reaction hydrolysisOrganic compound preparationCarboxylic acid esters separation/purificationDistillationOrganic synthesis
The invention belongs to the technical field of organic synthesis, and specifically relates to a continuous synthesis method of diethyl methylmalonate. 2-chloropropionic acid is continuously cyanidedto obtain 2-cyanopropionic acid; 2-cyanopropionic acid and ethanol carry out esterification reactions under the action of sulfuric acid to obtain a crude product of diethyl methylmalonate directly inone step; and the finished product is obtained after steps of washing and distillation. The reaction process is simple, and the yields of hydrolysis and esterification are high.
Owner:营创三征(营口)精细化工有限公司
Method of preparing (S)-(-)-2-chloropropionate and (R)-(+)-2-chloro propionic acid
InactiveCN100436399CThe reaction process is simpleHigh selectivityOrganic compound preparationCarboxylic acid esters preparationPropanoic acid2-Chloropropionic acid
A process for preparing (S)-(-)-2-chloropropionate and (R)-(+)-2-chloropropionic acid includes such steps as selective hydrolytic reaction of dl-2-chloropropionate in reaction medium under existence of pig steapsase, and separating two products from the resultant liquid.
Owner:ZHEJIANG UNIV OF TECH
Method for preparing purified 2-methyl-4-chloropropionic acid
InactiveCN104447288AHigh yieldReduce pollutionOrganic compound preparationCarboxylic acid salt preparationHypochlorite2-Chloropropionic acid
The invention provides a method for preparing purified 2-methyl-4-chloropropionic acid. The method comprises the following steps: carrying out a first reaction on o-cresol and a first alkaline compound to obtain an o-cresol salt; carrying out a second reaction on S-2 chloropropionic acid and a second alkaline compound to obtain a chloropropionic acid salt; carrying out a third reaction on the o-cresol salt and the chloropropionic acid salt in methylbenzene to obtain o-toluene oxypropionate; and under the action of an alkaline compound, carrying out a fourth reaction on a pypocholoride and the o-toluene oxypropionate to obtain the purified 2-methyl-4-chloropropionic acid. The optical content of the purified 2-methyl-4-chloropropionic acid prepared by the method provided by the invention is higher. In addition, the optical loss of the method provided by the invention in a preparation process of the purified 2-methyl-4-chloropropionic acid is smaller; moreover, the method provided by the invention can be used for preventing the generation of dioxin, to ensure a higher yield of the purified 2-methyl-4-chloropropionic acid; and the method for preparing purified 2-methyl-4-chloropropionic acid provided by the invention is simple in process and smaller in environmental pollution.
Owner:SHANDONG WEIFANG RAINBOW CHEM
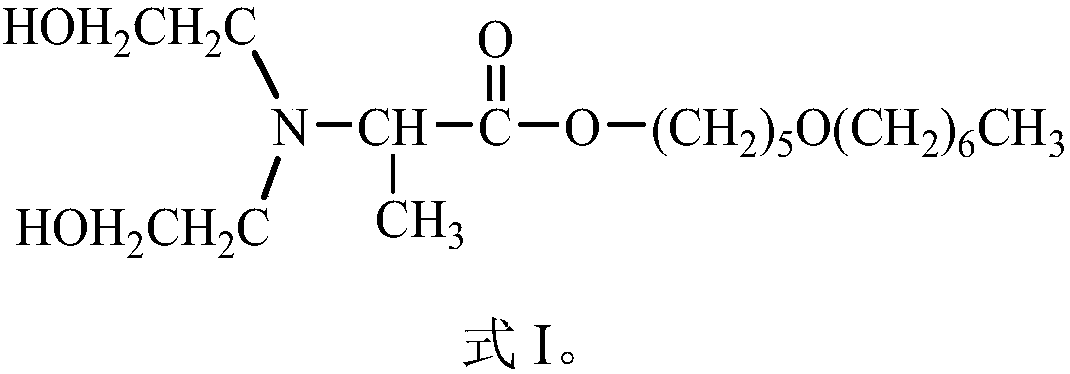
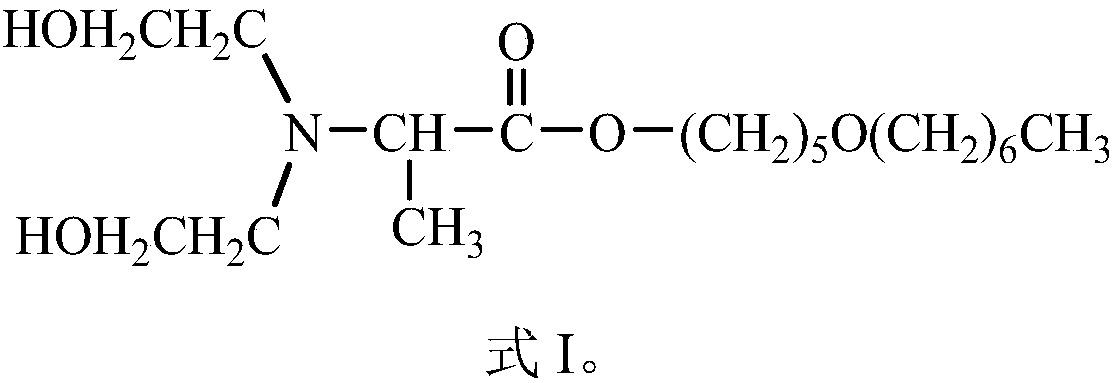
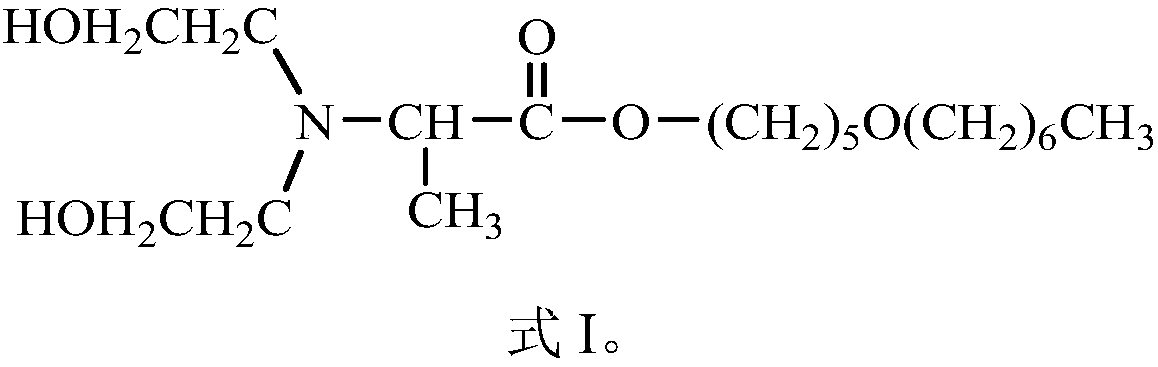
Compound Dahpe, preparation method and application thereof
InactiveCN108250093AImprove efficacyExcellent transdermal penetration enhancing activityOrganic active ingredientsOrganic compound preparation1-Pentanol2-Chloropropionic acid
Belonging to the field of chemical medicine, the invention in particular relates to a compound Dahpe, a preparation method and application thereof. The molecular structural formula of the compound Dahpe is shown as formula I in the specification. The preparation method of the compound Dahpe includes the steps of: acquiring the following three raw materials: 5-heptyloxy-1-pentanol, 2-chloropropionyl chloride and diethylamine alcohol; subjecting the 5-heptyloxy-1-pentanol and 2-chloropropionyl chloride to acylation reaction to obtain an intermediate 2-chloropropionic acid-5-heptyloxy-1-amyl ester; and subjecting the intermediate 2-chloropropionic acid-5-heptyloxy-1-amyl ester and the diethylamine alcohol to dechlorination amination reaction so as to obtain the compound Dahpe. The compound Dahpe can be applied to transdermal delivery preparations of cardiovascular and cerebrovascular drugs, significantly promote transdermal absorption of cardiovascular and cerebrovascular drugs, and enhance the efficacy of the drugs, and the transdermal penetration promoting activity is significantly superior to the prior art.
Owner:HANSHAN NORMAL UNIV
Industrial method for synthesizing 2-(4-methoxyphenoxy)-propionic acid through phase transfer
ActiveCN102010326BImprove conversion efficiencyGuaranteed conversion efficiencyOrganic compound preparationCarboxylic compound preparation4-MethoxyphenolPropanoic acid
Owner:SOUTH CHINA UNIV OF TECH
Synthesis method of high-purity D-2-chloropropionyl chloride
ActiveCN103408416BLower requirementOperational securityOrganic compound preparationCarboxylic compound preparationSynthesis methodsMethyl lactate
The invention provides a synthesis method of high-purity D-2-chloropropionyl chloride. A process path of chlorination, hydrolysis and chloroacylation of L-methyl lactate is adopted. Specifically, the synthesis method sequentially comprises the following steps: (1) the synthesis of D-methyl-2-chloropropionate: dropwise adding thionyl chloride into the mixed solution of the L-methyl lactate and pyridine, carrying out vacuum stirring, increasing the temperature to 55-90 DEG C, reacting for 1-5h, reducing the temperature to 25-35 DEG C, exhausting air, washing and separating, wherein the L-methyl lactate and the thionyl chloride are used as raw materials and the pyridine is used as a catalyst; (2) the synthesis of D-2-chloropropionic acid: hydrolyzing methyl-2-chloropropionate under the action of a sodium hydroxide solution, and carrying out chloroform extraction; and (3) the synthesis of D-2-chloropropionyl chloride: dropwise adding the thionyl chloride into the mixed solution of D-2-chloropropionic acid and the catalyst, increasing the temperature to 70-80 DEG C, reacting for 3-5 hours, and carrying out vacuum rectification to obtain a target product. The process path has the advantages that raw materials are easily available; synthesis steps are simple, safe and reliable; the waste gas generated in the production can be easily disposed; therefore, the process path is suitable for industrial production.
Owner:SHANDONG JINCHENG PHARMACEUTICAL GROUP CO LTD
Method for separating racemic 2-chloropropionic acid using capillary electrophoresis separation-diode array detection technology
InactiveCN104965018BChiral separation achievedEasy to operateMaterial analysis by electric/magnetic meansColor/spectral properties measurements2-Chloropropionic acidPeak area
Owner:HUBEI BIOCHEM PHARMA TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
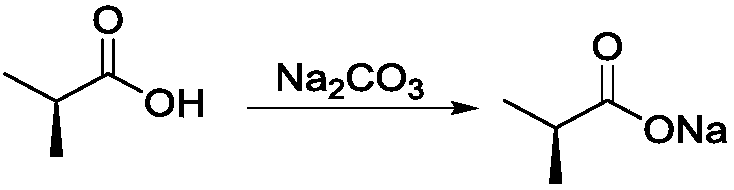

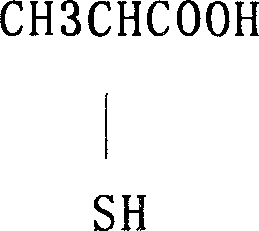
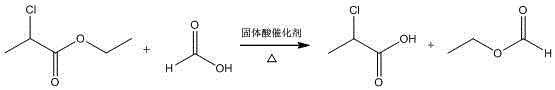
![[ethyl (R)-(+)-beta-(1- methylimidazolyl)-propionate]X chiral ionic liquid and synthesis method thereof [ethyl (R)-(+)-beta-(1- methylimidazolyl)-propionate]X chiral ionic liquid and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/24a02186-384f-4593-ba3d-529c35f11ce0/FSA00000436257600011.png)
![[ethyl (R)-(+)-beta-(1- methylimidazolyl)-propionate]X chiral ionic liquid and synthesis method thereof [ethyl (R)-(+)-beta-(1- methylimidazolyl)-propionate]X chiral ionic liquid and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/24a02186-384f-4593-ba3d-529c35f11ce0/BSA00000436257700022.png)
![Ethyl(2R)-2-[4-(4-cyano 2-fluorophenoxy)-phenoxy]propionic ester, and preparation and applications thereof Ethyl(2R)-2-[4-(4-cyano 2-fluorophenoxy)-phenoxy]propionic ester, and preparation and applications thereof](https://images-eureka.patsnap.com/patent_img/be006efb-75f9-4627-9fbe-d16b1f35cbdf/BDA0001345043830000021.png)
![Ethyl(2R)-2-[4-(4-cyano 2-fluorophenoxy)-phenoxy]propionic ester, and preparation and applications thereof Ethyl(2R)-2-[4-(4-cyano 2-fluorophenoxy)-phenoxy]propionic ester, and preparation and applications thereof](https://images-eureka.patsnap.com/patent_img/be006efb-75f9-4627-9fbe-d16b1f35cbdf/BDA0001345043830000051.png)
![Ethyl(2R)-2-[4-(4-cyano 2-fluorophenoxy)-phenoxy]propionic ester, and preparation and applications thereof Ethyl(2R)-2-[4-(4-cyano 2-fluorophenoxy)-phenoxy]propionic ester, and preparation and applications thereof](https://images-eureka.patsnap.com/patent_img/be006efb-75f9-4627-9fbe-d16b1f35cbdf/BDA0001345043830000052.png)

![Synthesis method of(R)-2-[4-(4-cyano-2-fluorophenoxy)phenoxy] propionic acid Synthesis method of(R)-2-[4-(4-cyano-2-fluorophenoxy)phenoxy] propionic acid](https://images-eureka.patsnap.com/patent_img/446ed8e5-d4c8-4b50-9ad0-3878a81f592f/842114DEST_PATH_IMAGE001.png)