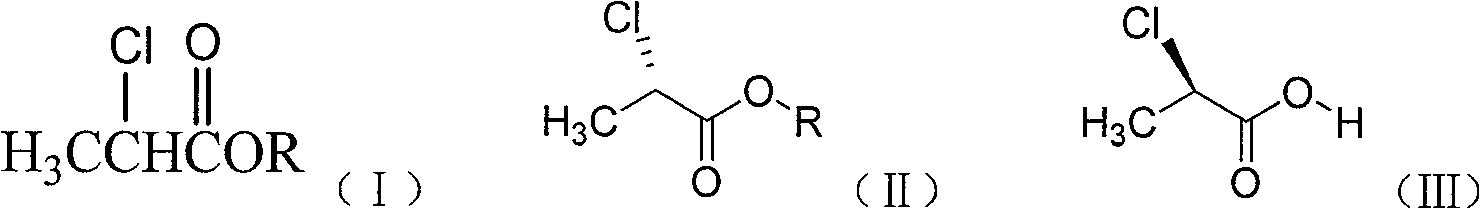
Method of preparing (S)-(-)-2-chloropropionate and (R)-(+)-2-chloro propionic acid
A technology of chloropropionic acid ester and chloropropionic acid is applied in chemical instruments and methods, preparation of carboxylic acid esters, preparation of organic compounds, etc., and can solve the problems of affecting splitting effect, low splitting yield and high production cost, Achieve the effect of low production cost, simple reaction process and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4
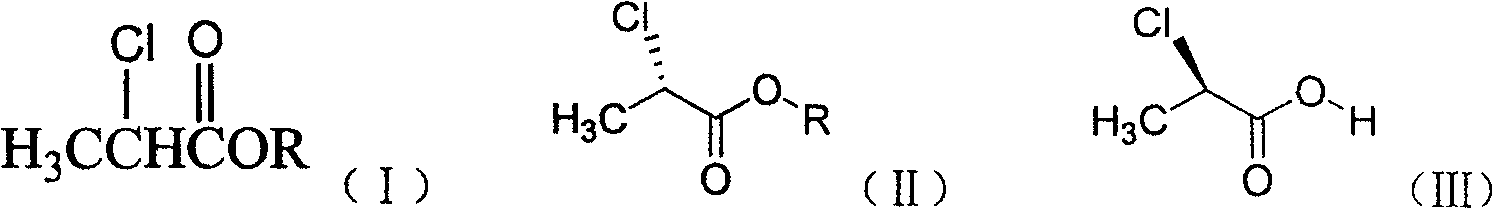
[0021] Add a certain amount of racemic 2-chloropropionate into a 500mL flask, 250mL 0.1mol / L disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution (pH=7.2), 1.0g of porcine pancreatic lipase, at 30°C Stir the reaction for a certain period of time. After the reaction, centrifuge to remove porcine pancreatic lipase, extract the reaction solution with dichloromethane, dry the organic layer over anhydrous sodium sulfate, and distill under reduced pressure to remove the organic solvent to obtain (S)-(-)-2-chloropropane Ester; the aqueous layer was acidified to pH less than or equal to 1.0 and extracted with dichloromethane, the organic layer was dried over anhydrous sodium sulfate, and the organic solvent was removed by vacuum distillation to obtain (R)-(+)-2-chloropropionic acid . The product yield and enantiomeric excess value determination results are listed in Table 1.
[0022] Table 1: Resolution results of different 2-chloropropionates
[0023]
[0024...
Embodiment 5
[0026] In a 500mL flask, add 8.23g of racemic butyl 2-chloropropionate, 250mL of 0.1mol / L disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution (pH=7.2), 1.0g of porcine pancreatic lipase, at 25°C The reaction was stirred for 14 hours. After the reaction, centrifuge to remove porcine pancreatic lipase, extract the reaction solution with dichloromethane, dry the organic layer over anhydrous sodium sulfate, and distill under reduced pressure to remove the organic solvent to obtain (S)-(-)-2-chloropropane Butyl acid 3.9 g (94.8% yield, 89% enantiomeric excess).
Embodiment 6
[0028] In a 500mL flask, add 4.12g of racemic butyl 2-chloropropionate, 250mL 0.1mol / L disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution (pH=7.2), 1.0g of porcine pancreatic lipase, at 30 The reaction was stirred at °C for 10 hours. After the reaction, centrifuge to remove porcine pancreatic lipase, extract the reaction solution with dichloromethane, dry the organic layer over anhydrous sodium sulfate, and distill under reduced pressure to remove the organic solvent (S)-(-)-2-chloropropionic acid Butyl ester 1.7 g (82.5% yield, 88.6% enantiomeric excess).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com