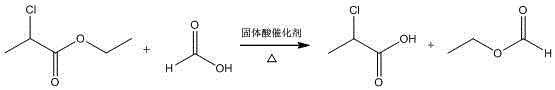Method for synthesizing (R)-2-chloropropionic acid by solid acid catalysis and trans-esterification reaction based on UIO-66
A UIO-66, solid acid catalyst technology, applied in chemical instruments and methods, carboxylate/lactone preparation, physical/chemical process catalysts, etc., can solve the problems of high industrial cost, low yield, environmental pollution, etc., To achieve the effect of being beneficial to environmental protection, less side reactions, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A method for the synthesis of (R)-2-chloropropionic acid based on UIO-66 solid acid catalyzed transesterification reaction of the present embodiment comprises the following steps:
[0024] (1) Solid acid catalyst SO 4 2- / Preparation of UIO-66
[0025] Weigh 0.053g ZrCl 4 and 0.034g of terephthalic acid were dissolved in 25mL of DMF (dimethylformamide), and then the above reactant was transferred to a polytetrafluoroethylene reactor, reacted at 120°C for 12h, filtered, and the precipitate was separated using DMF and Alternately washed with methanol, and dried the precipitate in an oven at 60°C for 12 hours to obtain UIO-66, then impregnated UIO-66 with 1.5mL sulfuric acid with a concentration of 1.5mol / L for 30min, filtered and dried, and placed in 500 The solid acid catalyst SO can be obtained in an oven at ℃ for 3 hours 4 2- / UIO-66;
[0026] (2) Preparation of (R)-2-chloropropionic acid
[0027] Add (R)-2-ethyl chloropropionate to the reactor, while stirring,...
Embodiment 2
[0030] A method for the synthesis of (R)-2-chloropropionic acid based on UIO-66 solid acid catalyzed transesterification reaction of the present embodiment comprises the following steps:
[0031] (1) Solid acid catalyst SO 4 2- / Preparation of UIO-66
[0032] Weigh 0.057gZrCl 4 and 0.034g of terephthalic acid were dissolved in 25mL of DMF (dimethylformamide), and then the above reactant was transferred to a polytetrafluoroethylene reactor, reacted at 110°C for 13h, filtered, and the precipitate was separated using DMF and Alternately washed with methanol, then dried the precipitate in an oven at 55°C for 13 hours to obtain UIO-66, then impregnated UIO-66 with 1.1mL of sulfuric acid with a concentration of 0.7mol / L for 30min, filtered and dried, and placed in 500 The solid acid catalyst SO can be obtained in an oven at ℃ for 3 hours 4 2- / UIO-66;
[0033] (2) Preparation of (R)-2-chloropropionic acid
[0034]Add (R)-2-ethyl chloropropionate to the reactor, while stirrin...
Embodiment 3
[0037] A method for the synthesis of (R)-2-chloropropionic acid based on UIO-66 solid acid catalyzed transesterification reaction of the present embodiment comprises the following steps:
[0038] (1) Solid acid catalyst SO 4 2- / Preparation of UIO-66
[0039] Weigh 0.055gZrCl 4 and 0.034g of terephthalic acid were dissolved in 25mL of DMF (dimethylformamide), and then the above reactant was transferred to a polytetrafluoroethylene reactor, reacted at 130°C for 11h, filtered, and the precipitate was separated using DMF and Alternately washed with methanol, and dried the precipitate in an oven at 65°C for 11 hours to obtain UIO-66, then impregnated UIO-66 with 2.2mL of sulfuric acid with a concentration of 1.5mol / L for 30min, filtered and dried, and placed in 500 The solid acid catalyst SO can be obtained in an oven at ℃ for 3 hours 4 2- / UIO-66;
[0040] (2) Preparation of (R)-2-chloropropionic acid
[0041] Add (R)-2-ethyl chloropropionate to the reactor, while stirrin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com