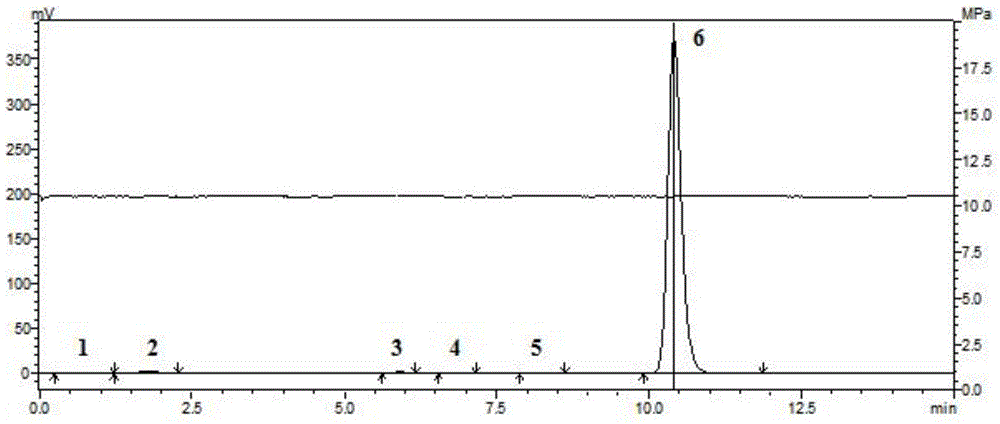
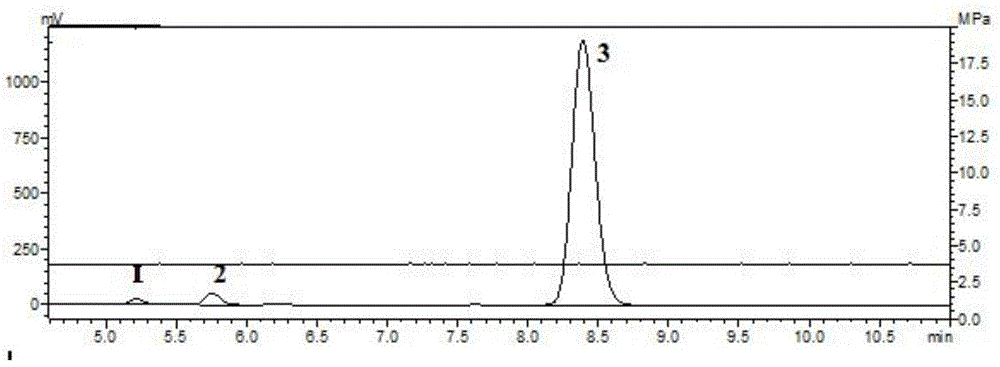
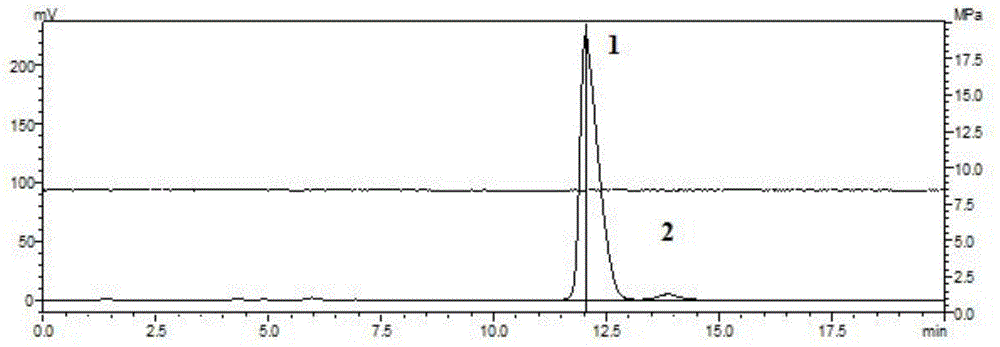
Method for preparing purified 2-methyl-4-chloropropionic acid
A technology of chloropropionic acid and chloropropionic acid, applied in the field of preparation of refined 2-methyl-4 chloropropionic acid, can solve the problems of large optical loss, low optical content of refined 2-methyl-4 chloropropionic acid, etc., and achieves low optical loss, Simple process and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The invention provides a kind of preparation method of refined 2-methyl-4-chloropropionic acid, comprising:
[0027] performing a first reaction with o-cresol and a first basic compound to obtain an o-cresol salt;
[0028] S-2 chloropropionic acid and the second basic compound are subjected to a second reaction to obtain chloropropionate;
[0029] Carrying out the third reaction of the o-formate and the chloropropionate in toluene to obtain o-tolyloxypropionic acid;
[0030] Under the action of amine compounds, the fourth reaction is carried out between hypochlorite and the o-tolyloxypropionic acid to obtain refined 2-methyl-4-chloropropionic acid.
[0031] The method provided by the present invention adopts o-cresol and chloropropionic acid to prepare o-cresyloxypropionic acid under alkaline conditions, and then chlorates the obtained o-cresyloxypropionic acid with hypochlorite to prepare refined 2,4 Chloropropionic acid. The optical content of the refined 2-methyl-...
Embodiment 1
[0075] Add 70.5g o-cresol and 82.5g mass concentration of 32.5% sodium hydroxide aqueous solution to a 250mL four-necked flask and carry out the first reaction at 40° C. for 1 hour to obtain sodium o-cresol.
[0076] Add 60.03g of S-2-chloropropionic acid and 66.5g of 32.5% sodium hydroxide aqueous solution to a 250mL four-necked flask at 30°C for 2 hours to obtain sodium chloropropionate.
[0077] Add 765 g of toluene to 153 g of the above-mentioned sodium o-cresolate for azeotropic dehydration to obtain a dehydrated product; add 50.6 g of the above-mentioned sodium chloropropionate dropwise to the dehydrated product, then raise the temperature to 130 ° C, and continue to drop The third reaction was carried out for 10 hours by adding 75.9 g of the above-mentioned sodium chloropropionate.
[0078] After the third reaction is completed, dilute the obtained third reaction product with 450g of water to obtain the material; transfer the material to a double-layer glass extraction ...
Embodiment 2
[0095] Add 70.4g of o-cresol and 82.3g of 32.5% sodium hydroxide aqueous solution to a 250mL four-necked flask at 40°C for 1 hour of the first reaction to obtain sodium o-cresol.
[0096] Add 60.04g of S-2-chloropropionic acid and 66.5g of 32.5% sodium hydroxide aqueous solution to a 250mL four-necked flask at 30°C for 2 hours to obtain sodium chloropropionate.
[0097] Add 758g of toluene to 152.7g of the above-mentioned sodium o-cresol and heat up to 100°C for dehydration to obtain a dehydrated product; add 43g of the above-mentioned sodium chloropropionate dropwise to the dehydrated product, and then raise the temperature to 128°C, Continue to dropwise add 83.5 g of the above-mentioned sodium chloropropionate to carry out the third reaction for 10 hours.
[0098] After the third reaction is completed, the obtained third reaction product is subjected to heat preservation treatment at 128° C. for 1 hour; the third reaction product after heat preservation treatment is diluted ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com