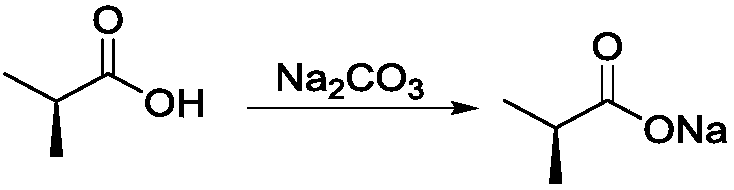Synthesis method of R-(+)-2-(4-hydroxyphenoxy) propionic acid
A technology of hydroxyphenoxy and synthesis methods, applied in chemical instruments and methods, separation/purification of carboxylic acid compounds, preparation of carboxylate salts, etc. Low recovery rate and other problems, to achieve the effect of easy availability of raw materials, simple synthesis route, and enhanced nucleophilic ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
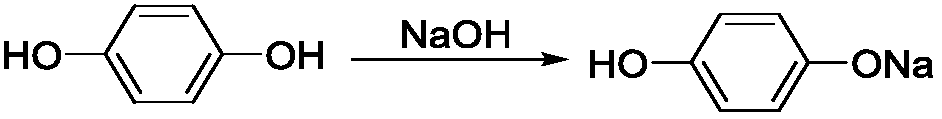
[0030] Add 4.2g NaOH (0.1mol) solid to a 100mL four-neck round bottom flask, add 30mL CH 3 OH, add the magnet, insert the internal temperature thermometer, install the spherical condenser, vacuumize, N 2 Under protection, add 11.1g of hydroquinone (0.1mol) in 3 batches, after the addition is complete, cool down to 18-20°C and react for 20min;
[0031] Weigh 5.6g of S-(-)-2-chloropropionic acid in a 100mL flask, add 20mL of CH 3 OH, make it dissolve completely, place the methanol solution of S-(-)-2-chloropropionic acid in an ice-water bath, slowly add 5.4g Na 2 CO 3 , stirred until no bubbles were generated to obtain S-(-)-2-chloropropionate sodium solution;
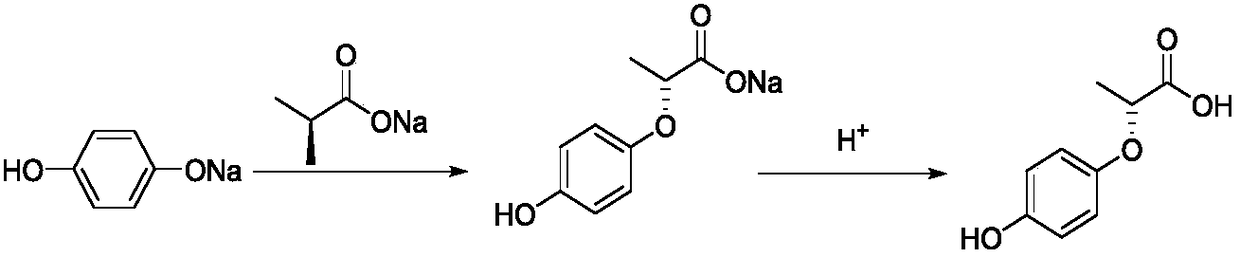
[0032] Slowly add the S-(-)-2-chloropropionate sodium solution dropwise into a four-neck round bottom flask, after the drop is complete, slowly raise the temperature to 30-32°C to continue the reaction. Monitor the reaction by TLC plate and HPLC, and stop the reaction until the raw material S-(-)-2-chloropropionic ac...
Embodiment 2
[0035] Add 6.2g NaOH (0.15mol) solid into a 100mL four-neck round bottom flask, add 30mL DMF, add a magnet, insert an internal temperature thermometer, install a spherical condenser, vacuumize, N 2 Under protection, add 11.1g of hydroquinone (0.1mol) in 3 batches, after the addition is complete, cool down to 14-16°C and react for 20min;
[0036] Weigh 5.6g of S-(-)-2-chloropropionic acid into a 100mL flask, add 20mL of DMF to dissolve it completely, place the S-(-)-2-chloropropionic acid DMF solution in an ice-water bath, and slowly add 8.0g Na 2 CO 3 , stirred until no bubbles were generated to obtain S-(-)-2-chloropropionate sodium solution;
[0037] Slowly add the S-(-)-2-chloropropionic acid DMF solution dropwise into the four-neck round bottom flask, after the drop is completed, slowly raise the temperature to 33-36°C to continue the reaction. Monitor the reaction by TLC plate and HPLC, and stop the reaction until the raw material S-(-)-2-chloropropionic acid is comple...
Embodiment 3
[0040] Add 5.2g of NaOH (0.125mol) solid into a 100mL four-neck round bottom flask, add 40mL of DMSO, add a magnet, insert an internal temperature thermometer, install a spherical condenser, and vacuumize. N 2 Under protection, add 13.9g hydroquinone (0.125mol) in 4 batches, after the addition is complete, cool down to 10-12°C and react for 25min;
[0041] Weigh 5.6g of S-(-)-2-chloropropionic acid into a 100mL flask, add 20mL DMSO to dissolve it completely, place the S-(-)-2-chloropropionic acid DMSO solution in an ice-water bath, and slowly add 5.4g Na 2 CO 3 , stirred until no bubbles were generated to obtain S-(-)-2-chloropropionate sodium solution;
[0042] Slowly add the S-(-)-2-chloropropionic acid DMSO solution dropwise into the four-neck round bottom flask, after the drop is completed, slowly raise the temperature to 38-40°C to continue the reaction. Monitor the reaction by TLC plate and HPLC, and stop the reaction until the raw material S-(-)-2-chloropropionic ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com