Method for preparing chiral (S)-2-propionic acid
A chloropropionic acid and chiral technology, applied in the field of preparation of chiral chloropropionic acid, can solve problems such as high product cost, waste of water sources, pollution, etc., and achieve the advantages of improving market competitiveness, reducing production costs, and improving industrialization prospects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]
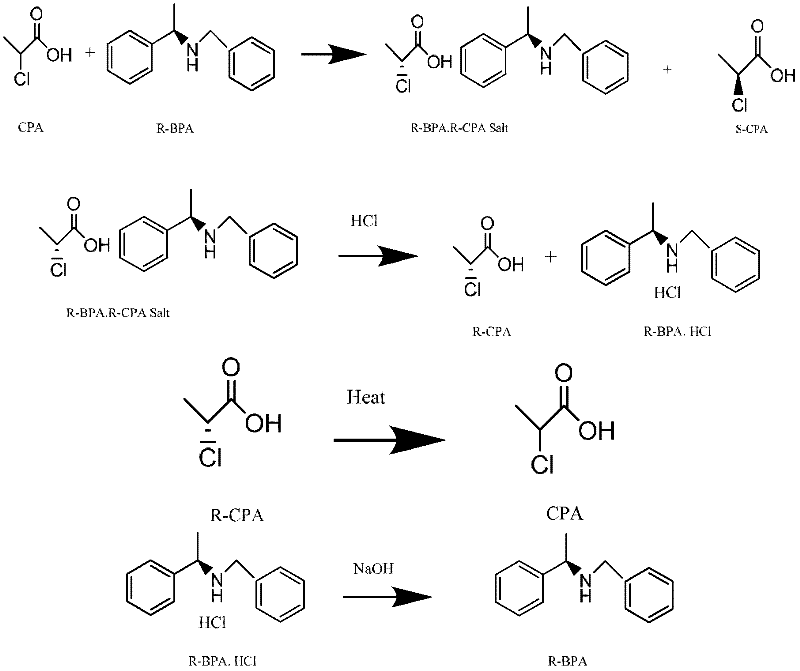
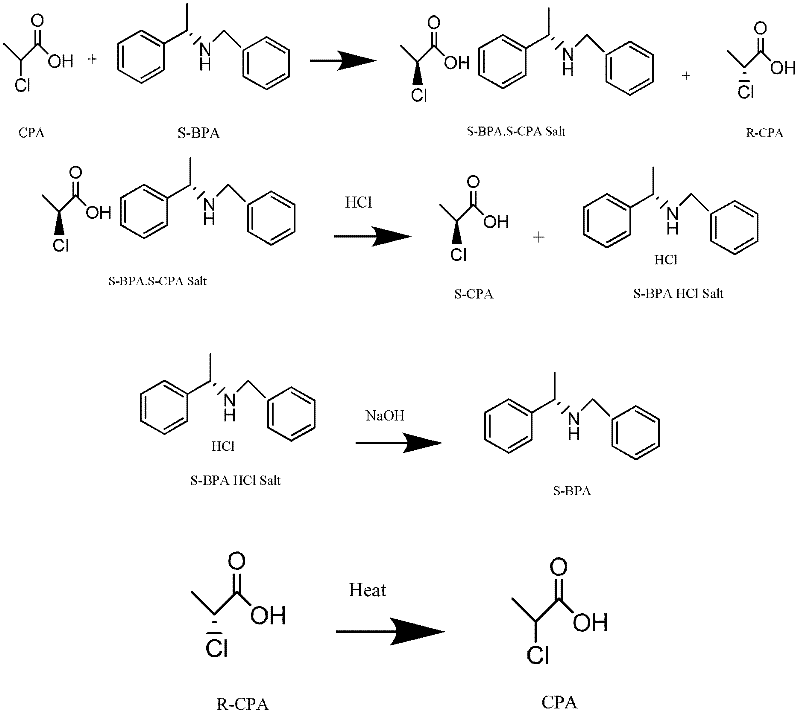
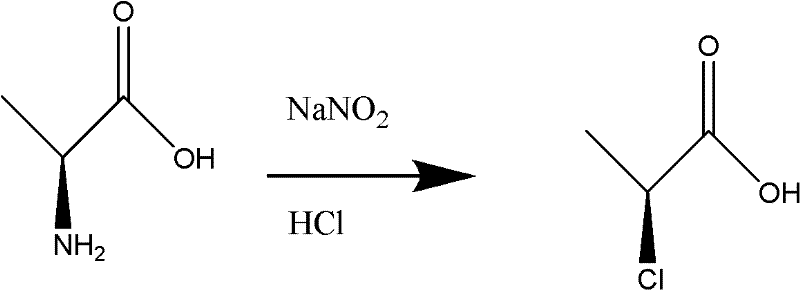
[0039] Resolution of (S)-2-chloropropionic acid: 118.5 grams of 2-chloropropionic acid (1mol) was added to 1000 milliliters of absolute ethanol, and under stirring, 116.1 grams of (R)-N-benzyl-phenethylamine was added dropwise (0.55mol) mixed with 200ml ethanol, drop it within 1 hour, then heat it to reflux within 1 hour, keep it under reflux for 30 minutes; then cool it down to room temperature naturally, then turn it to an ice-water bath to cool at 0-5°C, and keep it at this temperature for 5 At this time, a large amount of (R)-2-chloropropionic acid · (R)-N-benzyl-phenethylamine salt was precipitated; filtered, and the filter cake was washed with a small amount of ice ethanol; the filtrate was collected, and the ethanol was distilled under reduced pressure to dryness. (S)-2-chloropropionic acid was distilled under high vacuum by the oil change pump to obtain 48.6 grams of product (S)-2-chloropropionic acid. Analytical detection of chemical purity: 99.45% (GC), c...
Embodiment 2
[0041]
[0042]Racemization reclaims 2-chloropropionic acid: filter cake (R)-2-chloropropionic acid · (R)-N-benzyl-phenethylamine salt (approximately 200 grams of wet product) in Example 1 is added to 600ml In isopropanol, pass through dry hydrogen chloride gas until the pH value is 2~3, and (R)-2-chloropropionic acid is freed; at this time, a large amount of (R)-N-benzyl-phenethylamine hydrochloride precipitates Precipitate, filter, and wash the filtrate with a small amount of isopropanol; heat the filtrate to reflux, sample and analyze until no optical activity is detected; after distilling isopropanol under reduced pressure, continue to distill about 55.3 grams of 2-chloropropionic acid by raising the temperature. The 2-chloropropionic acid recovered by racemization can be separated and recycled next time.
Embodiment 3
[0044]
[0045] Reclaim resolution reagent (R)-N-benzyl-phenethylamine: filter cake (R)-N-benzyl-phenethylamine hydrochloride (about 146 grams of wet product) in embodiment 2 is added 500ml di In methyl chloride, add 25 grams of caustic soda in batches to pH 12-14; filter, wash the filter cake with a small amount of dichloromethane, and distill the filtrate under reduced pressure to recover the dichloromethane solvent; high vacuum distillation (R)-N-benzyl- Phenylethylamine, about 103.8 grams. The reclaimed (R)-N-benzyl-phenethylamine can be recycled for next time resolution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Chemical purity | aaaaa | aaaaa |
| Chiral purity | aaaaa | aaaaa |
| Chemical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com