Compound Dahpe, preparation method and application thereof
A compound, the technology of heptyloxy, which is applied in the preparation of organic compounds, cyanide reaction preparation, chemical instruments and methods, etc., can solve the problems of limited application of transdermal absorption enhancers and low yield, and achieve the promotion of transdermal absorption , Yield increase, enhance the efficacy of the drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] On the other hand, the embodiment of the present invention also provides a preparation method of the above-mentioned compound Dahpe, the preparation method comprising the following steps:
[0020] S01: Obtain three raw materials of 5-heptyloxy-1-pentanol, 2-chloropropionyl chloride and diethylaminol;
[0021] S02: acylating 5-heptyloxy-1-pentanol and 2-chloropropionyl chloride in step S01 to obtain intermediate 2-chloropropionic acid-5-heptyloxy-1-pentyl ester;
[0022] S03: Dechlorination of the intermediate 2-chloropropionic acid-5-heptyloxy-1-pentyl ester in step S02 and diethylamino alcohol in step S01 to obtain the compound Dahpe of the above-mentioned invention embodiment .
[0023] The reaction process of the above-mentioned preparation method is as follows. The preparation method not only has simple process, easy operation and low cost, but also has no pollution to the environment during the preparation process. Compared with the preparation method of the prio...
Embodiment 1
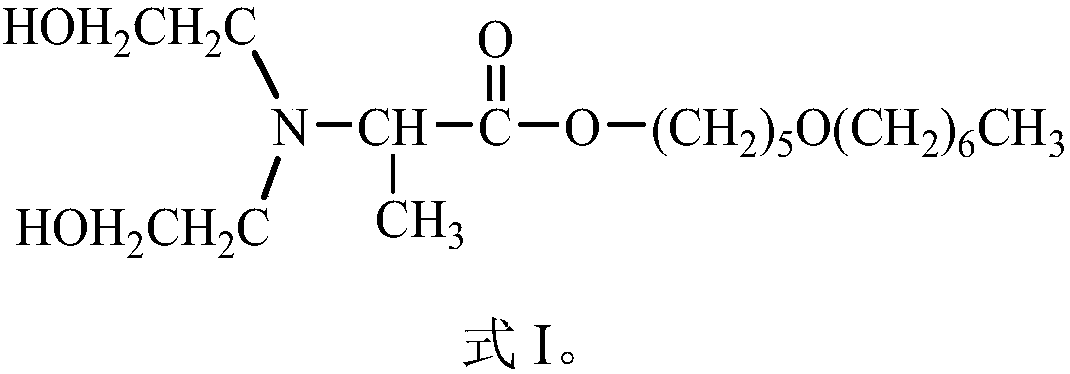
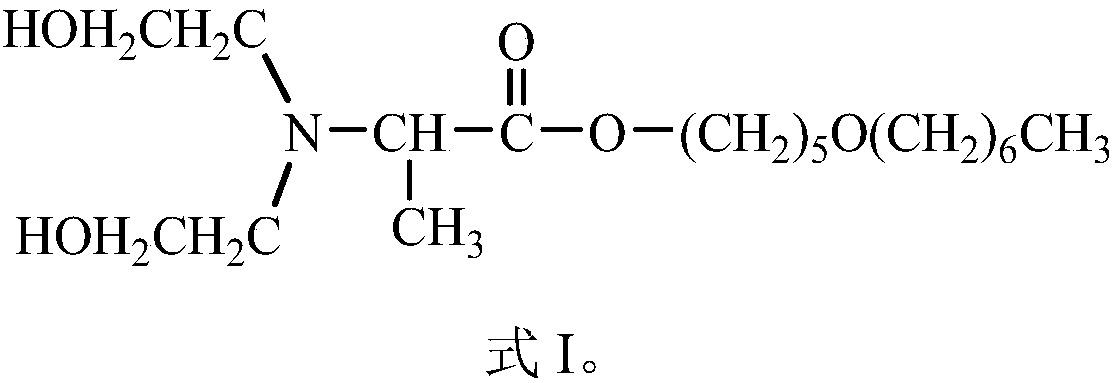
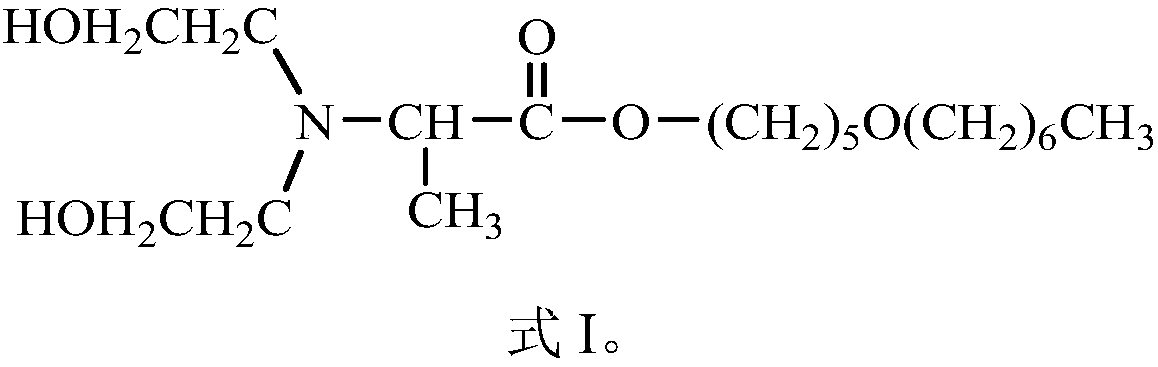
[0044] This embodiment provides a compound Dahpe, whose chemical name is: 2-(N,N-dihydroxyethyl)alanine-5-heptyloxy-1-pentyl ester; the structural formula is shown in the aforementioned formula I.
[0045] The preparation method of above-mentioned compound Dahpe is as follows:
[0046] S11: Obtain three raw materials of 5-heptyloxy-1-pentanol, 2-chloropropionyl chloride and diethylaminol;
[0047] S12: Acylation reaction of 5-heptyloxy-1-pentanol and 2-chloropropionyl chloride to obtain intermediate 2-chloropropionic acid-5-heptyloxy-1-pentyl ester;
[0048] S13: Dechlorination of the intermediate 2-chloropropionic acid-5-heptyloxy-1-pentyl ester and diethylamino alcohol was carried out to obtain the compound Dahpe of the above-mentioned embodiment of the invention.
[0049] Concrete above-mentioned step S12 acylation reaction, the synthesis process of intermediate 2-chloropropionic acid-5-heptyloxy-1-pentyl ester is:
[0050] S121: In a 50mL three-neck flask, place 9.47g (4...
Embodiment 2
[0059] This embodiment provides a compound Dahpe, whose chemical name is: 2-(N,N-dihydroxyethyl)alanine-5-heptyloxy-1-pentyl ester; the structural formula is shown in the aforementioned formula I.
[0060] The preparation method of above-mentioned compound Dahpe is as follows:
[0061] S21: Obtain three raw materials of 5-heptyloxy-1-pentanol, 2-chloropropionyl chloride and diethylaminol;
[0062] S22: Acylation reaction of 5-heptyloxy-1-pentanol and 2-chloropropionyl chloride to obtain intermediate 2-chloropropionic acid-5-heptyloxy-1-pentyl ester;
[0063] S23: Dechlorination of the intermediate 2-chloropropionic acid-5-heptyloxy-1-pentyl ester and diethylamino alcohol was carried out to obtain the compound Dahpe of the above-mentioned embodiment of the invention.
[0064] Concrete above-mentioned step S22 acylation reaction, the synthesis process of intermediate 2-chloropropionic acid-5-heptyloxy-1-pentyl ester is:
[0065] S221: In a 50mL three-necked flask, place 8.68g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com