Method for synthesizing fenoxanil
A technology of blastamide and toluene, which is applied in the field of synthesis of blastamide, can solve the problems that are not conducive to popularization and application, long reaction time, air pollution, etc., and achieve the effects of reducing the types of raw materials, shortening the reaction cycle, and reducing the cost of solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
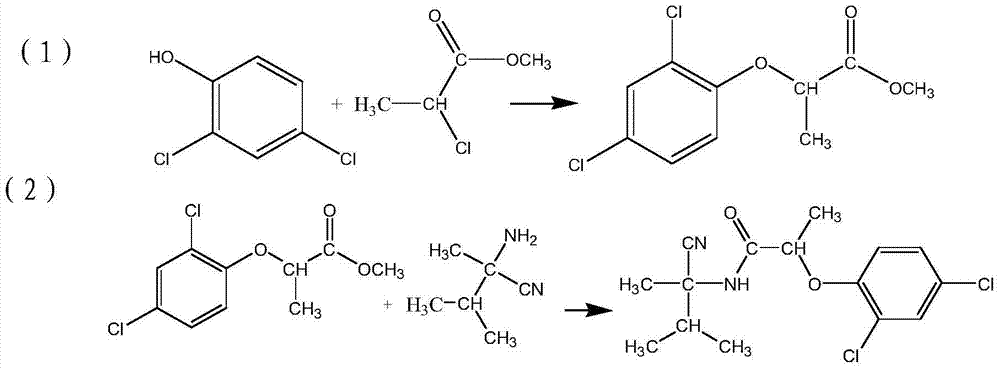
[0018] Add 163g (1mol) of 2,4-dichlorophenol, 1L of toluene, 40g (1mol) of sodium hydroxide and 122.5g (1mol) of methyl 2-chloropropionate to a 2L reaction flask, and raise the temperature to 55°C to react 8 hour, after the reaction finishes, wash the toluene phase with water, and the toluene phase after the water wash is carried out decompression distillation solvent to obtain methyl propionate;
[0019] Add 112g (1mol) of 2-amino-2,3-dimethylbutyronitrile, 550ml of toluene, 84g (1mol) of sodium bicarbonate and the methyl propionate obtained from the reaction to a 1L reaction flask, and control the temperature at 15°C. React for 6 hours, wash the toluene phase with water after the reaction is completed, and carry out the solvent distillation of the toluene phase after the water washing, and then recrystallize with a mixed solvent of 1.1L water and ethanol, and the volume ratio of the ethanol and water is 1:1 , down to room temperature, filtered to obtain blastamide 325g, yiel...
Embodiment 2
[0021] Add 163g (1mol) 2,4-dichlorophenol, 1.2L toluene, 40g (1mol) sodium hydroxide and 134.8g (1.1mol) methyl 2-chloropropionate to a 2L reaction flask, and raise the temperature to 60°C After reacting for 9 hours, the toluene phase was washed with water after the reaction, and the washed toluene phase was subjected to vacuum distillation to obtain methyl propionate;
[0022] Add 112g (1mol) of 2-amino-2,3-dimethylbutyronitrile, 500ml of toluene, 84g (1mol) of sodium bicarbonate and the methyl propionate obtained from the reaction to a 1L reaction flask, and control the temperature at 10°C. React for 5 hours, wash the toluene phase with water after the reaction is finished, carry out the solvent distillation of the toluene phase after the water washing, and then carry out recrystallization with a mixed solvent of 1L water and ethanol, and the volume ratio of the ethanol and water is 1:1.2, After cooling down to room temperature, 326 g of blastamide was obtained by filtration...
Embodiment 3
[0024] Add 163g (1mol) 2,4-dichlorophenol, 1.3L toluene, 40g (1mol) sodium hydroxide and 147g (1.2mol) methyl 2-chloropropionate to a 2L reaction flask, and heat up to 45°C for reaction After 10 hours, the toluene phase was washed with water after the reaction, and the washed toluene phase was subjected to vacuum distillation to obtain methyl propionate;
[0025] Add 112g (1mol) of 2-amino-2,3-dimethylbutyronitrile, 550ml of toluene, 84g (1mol) of sodium bicarbonate and the methyl propionate obtained from the reaction into a 1L reaction flask, and control the temperature at 8°C. React for 7 hours, wash the toluene phase with water after the reaction is completed, and carry out the solvent distillation of the toluene phase after the water washing, and then recrystallize with a mixed solvent of 1.2L water and ethanol, and the volume ratio of the ethanol and water is 1:1.4 , down to room temperature, filtered to obtain blastamide 330g, yield 95.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com