Synthetic method of D-(+)-2-chloro-propanoyl chloride
The technology of a chloropropionyl chloride and a synthesis method, which is applied in the synthesis field of D--2-chloropropionyl chloride, can solve problems such as low product yield, and achieve high product yield, novel reaction catalyst and no environmental impact. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
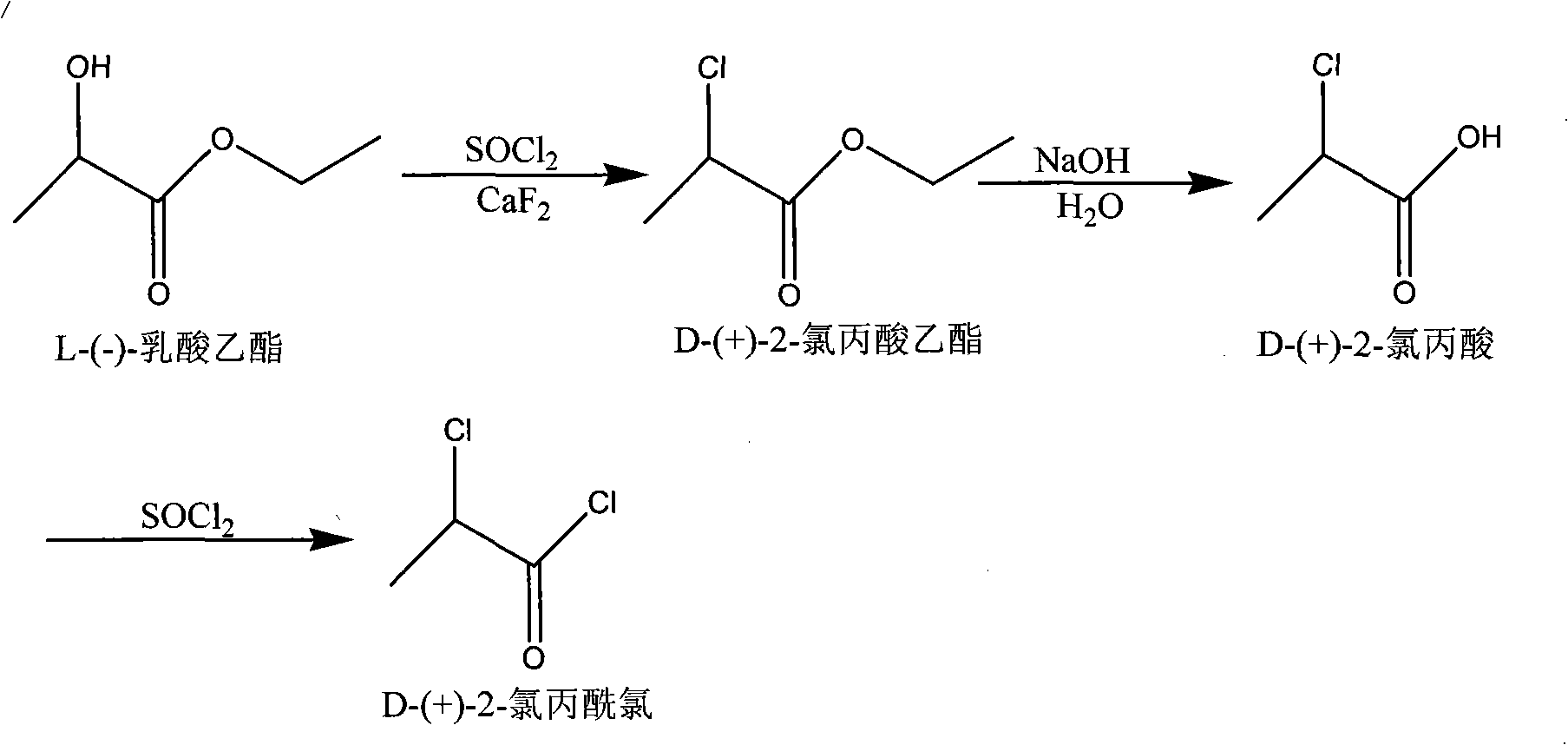
[0018] Step a, synthetic D-(+)-2-chloropropionic acid ethyl ester
[0019] A four-necked reaction flask equipped with mechanical stirring and a hydrogen chloride absorber was installed, 125 grams of thionyl chloride and 10 grams of calcium fluoride catalyst were added, and 100 grams of L-ethyl lactate was added dropwise at room temperature (actually 27° C.). After dripping, heat up to 65-70°C, stir at constant temperature for 2 hours, heat up to 168°C for reflux reaction for 15 hours, stop the reaction, cool the reaction solution to room temperature (actually 27°C), enter the rectification tank for rectification to obtain D -(+)-2-Ethyl chloropropionate, the yield is 89.6%, the purity of the product reaches 99.5%, the optical purity of the chiral GC analysis reaches 97.1%, and the optical rotation of the product is +19.2 degrees.
[0020] Step b, synthesis of D-(+)-2-chloropropionic acid
[0021] Set a four-necked reaction flask equipped with mechanical stirring, add 70 grams...
Embodiment 2
[0025] Step a, synthetic D-(+)-2-chloropropionic acid ethyl ester
[0026] Set up a reaction kettle equipped with mechanical stirring and a hydrogen chloride absorber, add 240 kg of thionyl chloride and 10 kg of calcium fluoride catalyst, start stirring, and at room temperature (actually 25°C), feed at a rate of 5 kg / min , add 200 kg of L-ethyl lactate, after adding L-ethyl lactate, heat up to 65-70°C and stir for 3 hours, then heat up to 170°C for reflux reaction for 15 hours, stop the reaction, cool to room temperature (actually 25 ℃), enter the rectifying kettle and carry out rectification to obtain the D-(+)-2-chloropropionic acid ethyl ester product, the productive rate is 79.7%, the purity of the product reaches 99.5%, and the chiral GC analysis optical purity reaches 97.1%, The optical rotation of the product is +19.2 degrees.
[0027] Step b, synthesis of D-(+)-2-chloropropionic acid
[0028] Set up the reactor that mechanical stirring is housed, add 140 kilograms of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical rotation | aaaaa | aaaaa |
| Optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com