Synthesis method of N-(2-chloride)-propionyl-glutamine
A technology of glutamine and synthetic method, which is applied in the field of synthesis of N-(2-chloro)-propionyl-glutamine, can solve the problems of low yield and long route, and achieve high product yield and simple process Feasible, simple and novel synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
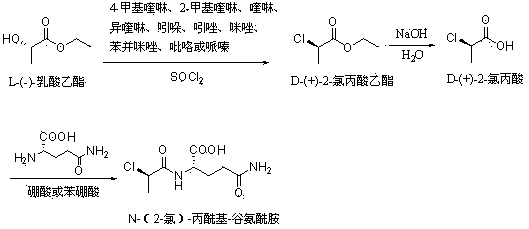
[0018] Step a, synthetic D-(+)-2-chloropropionic acid ethyl ester
[0019] Take 250 g (2.12 moles) of L-ethyl lactate in a dry three-necked bottle, o Add 2 grams of catalyst 2-methylquinoline (or 4-methylquinoline, quinoline, isoquinoline, indole, indazole, imidazole, benzimidazole, pyrrole, piperazine) below C, and then drop 262 g (2.2 moles) of thionyl chloride, keeping the temperature at 10 o Below C, after the dropwise addition, at 5 o Stir for 1 hour below C, then raise the temperature to 50 o C , stirred for 3 hours, cooled the reaction solution to 30 o C. HCl, SO2, and SOCl2 were removed under reduced pressure, and rectification was carried out after the gas was exhausted to obtain D-(+)-2-chloropropionic acid ethyl ester product with a yield of 96%. The product purity reaches 99.8%, and the optical purity reaches 99.5%.
[0020] Step b, synthesis of D-(+)-2-chloropropionic acid
[0021] Take 100 grams (0.73 moles) of ethyl D-(+)-2-chloropropionate, at 0 o St...
Embodiment 2
[0026] Step a, synthetic D-(+)-2-chloropropionic acid ethyl ester
[0027] Take 530 grams (4.45 moles) of thionyl chloride in a dry three-necked flask, o Add dropwise 500 grams (4.24 moles) of L-ethyl lactate and 4 grams of catalyst 2-methylquinoline (or 4-methylquinoline, quinoline, isoquinoline, indole, indazole, imidazole, benzene and imidazole, pyrrole, piperazine) mixture, keep the temperature at 10 o Below C, after the dropwise addition, at 5 o Stir for 1 hour below C, then raise the temperature to 50 o C , stirred for 5 hours, cooled the reaction solution to 30 o C. HCl, SO2, and SOCl2 were removed under reduced pressure, and rectification was carried out after the gas was exhausted to obtain D-(+)-2-chloropropionic acid ethyl ester product with a yield of 93%. The product purity reaches 99.5%, and the optical purity reaches 98.5%.
[0028] Step b, synthesis of D-(+)-2-chloropropionic acid
[0029] Take 500 grams (3.66 moles) of ethyl D-(+)-2-chloropropionate, at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com