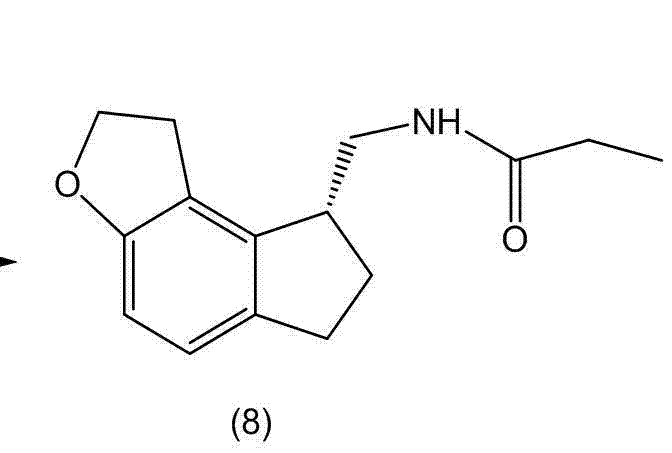
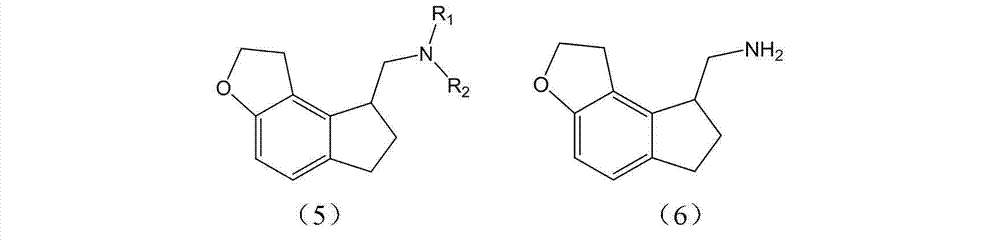
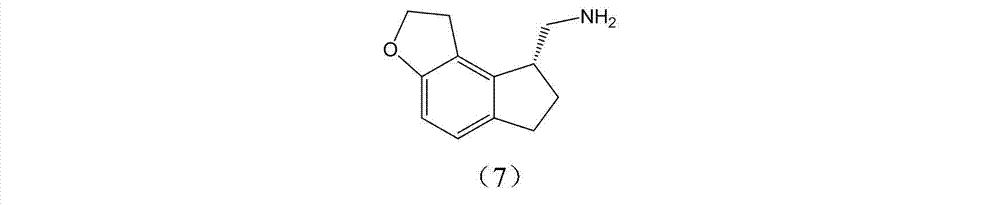
Preparation method and intermediate of ramelteon
A technology of ramelteon and reaction time, applied in the preparation of ramelteon and the field of intermediates thereof, can solve the problems of being difficult to obtain, unfavorable to meet the quality requirements of industrialized production products, expensive metal complex catalysts, etc. Low cost, guaranteed quality and safety, good product stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of 2-(1,6,7,8-tetrahydro-2H-indeno-[5,4-b]furan-8-ene)-N-phthalimide
[0044] Add N-(2-triphenylphosphine)phthalimide bromide salt (227g, 0.44mol) and anhydrous tetrahydrofuran (185mL) into the reaction flask, add 60% sodium hydride (19.2g , 0.44mol), after the addition was completed, the reaction was stirred at room temperature for 1h. After the reaction, the above reaction solution was added dropwise into 280 mL tetrahydrofuran solution containing 1,2,6,7-tetrahydro-8H-indeno[5,4b]furan-8-one (70g, 0.4mol), dropwise After completion, react at room temperature until the reaction of the raw material 1,2,6,7-tetrahydro-8H-indeno[5,4b]furan-8-one is complete, add 200mL water to terminate the reaction, and wash the aqueous layer with ethyl acetate (100 mLx3) Extracted, combined organic layers, washed with saturated brine (100 mLx3), dried over anhydrous sodium sulfate, filtered filtrate was evaporated to dryness to obtain 96.5 g of white solid, yield 72.5%.
Embodiment 2
[0046] Preparation of 2-(1,6,7,8-tetrahydro-2H-indeno-[5,4-b]furan-8-yl)-N-phthalimide
[0047] Add 2-(1,6,7,8-tetrahydro-2H-indeno-[5,4-b]furan-8-ene)-N-phthalimide (53g, 0.4mol), Raney nickel (10g) and saturated ammonia ethanol solution (1L) were added to a 3 L autoclave, hydrogen was introduced to 4MPa, heated to 40°C and stirred for 5 hours, filtered, and the filtrate was evaporated to dryness to obtain Yellow solid 49.4g, yield 92.6%.
Embodiment 3
[0049] Preparation of 2-(1,6,7,8-tetrahydro-2H-indeno-[5,4-b]furan-8-yl)ethylamine
[0050] Add 2-(1,6,7,8-tetrahydro-2H-indeno-[5,4-b]furan-8-yl)-N-phthalimide (43g, 0.13mol) and ethanol (1L), add hydrazine hydrate (19g, 0.39mol) dropwise at room temperature, react at 70°C for 8h after the dropwise addition, filter, wash the colloidal solid with ethyl acetate, collect the filtrate and evaporate to dryness to obtain light yellow Oil (22.5g, 92.1%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com