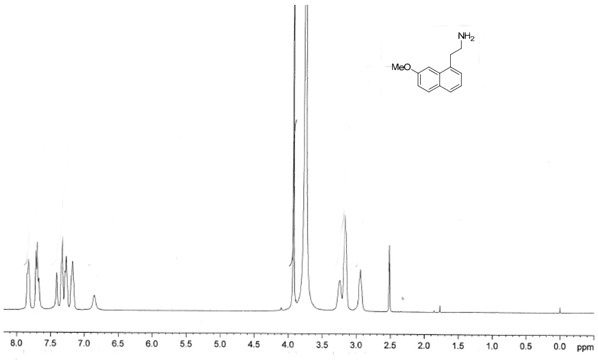
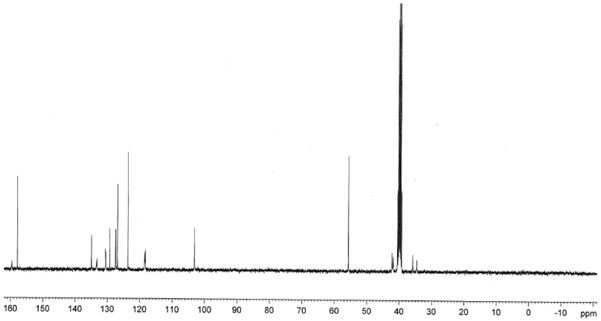
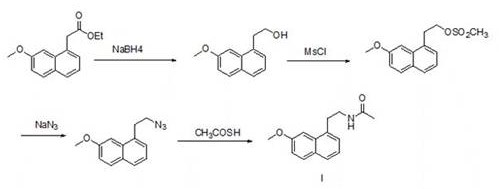
Preparation method of agomelatine intermediate
A technology for an intermediate and a synthesis method, which is applied in the field of preparation of agomelatine intermediates, can solve the problems of high difficulty in industrialization, harsh reaction conditions, long reaction routes, etc., and achieves simple and safe reaction operation, mild reaction conditions, and reaction short step effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: A kind of preparation method of agomelatine intermediate
[0050] 1. Acid chloride of 7-methoxy-1-naphthylpropionic acid
[0051]In a 1L glass reaction flask, add 863.40 g of methylene chloride and 57.56 g (0.25 mol) of 7-methoxy-1-naphthylpropionic acid in sequence, and raise the temperature to 35°C until 7-methoxy-1-naphthyl All propionic acid was dissolved. Control the temperature at 35°C, and slowly add 59.48g (0.5mol) of thionyl chloride dropwise to the reaction system under the state of slight reflux. The dropwise addition time is 2 hours. The reaction was continued for 3.5 hours. After the reaction was completed, dichloromethane and excess thionyl chloride were distilled off under reduced pressure to obtain 7-methoxy-1-naphthylpropionyl chloride.
[0052] 2. Amidation
[0053] All the 7-methoxyl-1-naphthyl propionyl chloride obtained in step 1 was dissolved in 460 grams of ethyl acetate, and the reaction system was kept at 0°C under an ice-water...
Embodiment 2
[0059] Embodiment 2: A kind of preparation method of agomelatine intermediate
[0060] 1. Acid chloride of 7-methoxy-1-naphthylpropionic acid
[0061] In a 1L glass reaction flask, add 575.6 grams of methylene chloride and 57.56 g (0.25 mol) of 7-methoxy-1-naphthyl propionic acid in sequence, and heat up to 30°C until 7-methoxy-1-naphthalene All propionic acid was dissolved. Control the temperature at 30°C and slowly add 29.74 g (0.25 mol) of thionyl chloride to the reaction system dropwise under the state of slight reflux. The dropwise addition time is 1 hour. The reaction was continued for 2 hours. After the reaction was completed, the solvent and excess thionyl chloride were distilled off under reduced pressure to obtain 7-methoxy-1-naphthylpropionyl chloride.
[0062] 2. Amidation
[0063] Dissolve all the 7-methoxy-1-naphthylpropionyl chloride obtained in step 1 in 345.36 grams of ethyl acetate, and keep the temperature of the reaction system at -5°C in an ice-water b...
Embodiment 3
[0069] Embodiment 3: A kind of preparation method of agomelatine intermediate
[0070] 1. Acid chloride of 7-methoxy-1-naphthylpropionic acid
[0071] 1151.20 g of dichloromethane and 57.56 g (0.25 mol) of 7-methoxy-1-naphthylpropionic acid were successively added to a 2 L glass reaction flask, and the temperature was raised to 40°C until complete dissolution. Control the temperature at 40°C and add 118.97 g (1 mol) of thionyl chloride slowly to the reaction system in a micro-reflux state. The dropwise addition time is 4 hours. During the dropping process, keep the system in a micro-reflux state. React for 6 hours. After the reaction was completed, the solvent and excess thionyl chloride were distilled off under reduced pressure to obtain 7-methoxy-1-naphthylpropionyl chloride.
[0072] 2. Amidation
[0073] Dissolve all the 7-methoxy-1-naphthylpropionyl chloride obtained in step 1 in 575.6 grams of ethyl acetate, keep the temperature of the reaction system at 5°C in an ice...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com