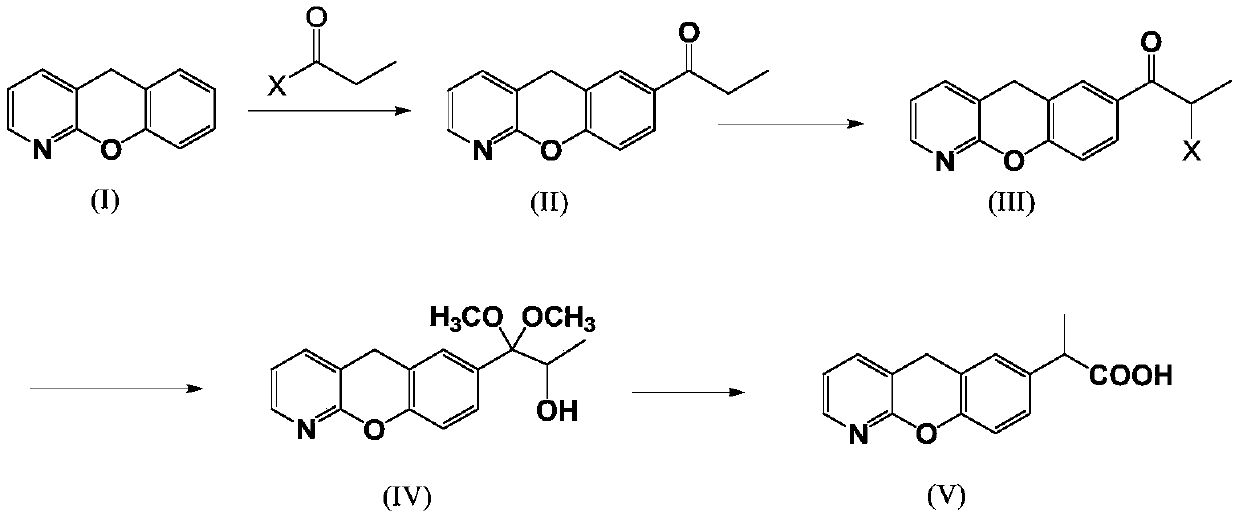
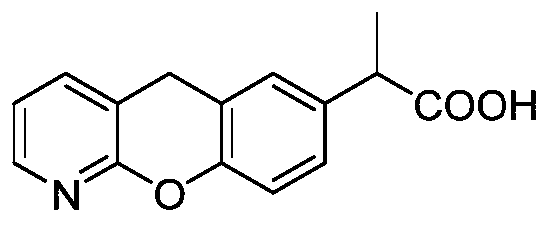
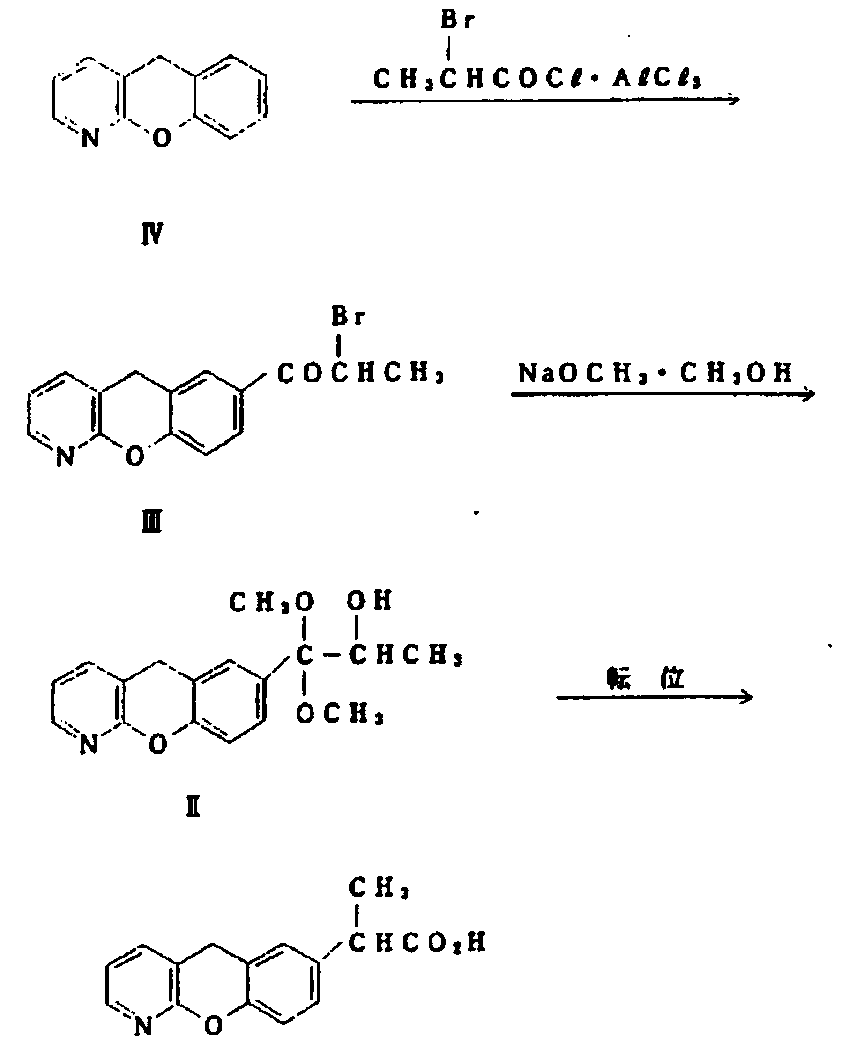
Novel preparation method of pranoprofen
An intermediate and equivalent ratio technology, which is applied in the field of preparation of propionate non-steroidal anti-inflammatory drug pranoprofen, can solve the problems of low yield and reduction, and achieve simple operation, cost reduction and increased yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] step one)
[0061] Add 5H-[1]-benzopyran[2,3-b]pyridine (50g, 0.27mol) into a one-necked flask, then add 250ml of dichloromethane, cool to 0°C, add anhydrous aluminum trichloride ( 108g, 0.81mol), and finally propionyl chloride (37g, 0.4mol) was slowly added dropwise, and the dropwise addition was completed in 15 minutes. Adjust the pH to about 4 with sodium, filter, add 250ml of dichloromethane to the filtrate for extraction, add water to the organic layer after layering, and spin dry the organic layer to obtain 57g of a light red solid, with a yield of 89%.
[0062] Step (2)
[0063] Add 7-(1-propionyl)-5H-[1]-benzopyran[2,3-b]pyridine (50g, 0.208mol) into the one-necked flask, then add 150ml of carbon tetrachloride, and then add NBS (44g, 0.24mol) was heated and refluxed for 5h. After the reaction was completed, water was added, washed twice with 250ml of water, separated into layers, and the organic layer was spin-dried to obtain a reddish-brown solid 7-(2-bromopr...
Embodiment 2
[0069] step one)
[0070] Add 5H-[1]-benzopyran[2,3-b]pyridine (50g, 0.27mol) into a one-necked flask, then add 150ml of ethyl acetate, cool to -5°C, add anhydrous aluminum chloride (72g, 0.54mol), and finally propionyl bromide (36g, 0.27mol) was slowly added dropwise, and the dropwise addition was completed in 10 minutes. The temperature control during the dropwise addition was ≤10°C, and the temperature was raised to room temperature to continue the reaction for 2 hours, and then the system was added to 250ml of ice water , adjust the pH to about 4 with sodium carbonate, filter, add 250ml of dichloromethane to the filtrate for extraction, add water to the organic layer after layering, spin dry the organic layer to obtain 55g of a light red solid, and the yield is 85%.
[0071] Step (2)
[0072] Add 7-(1-propionyl)-5H-[1]-benzopyran[2,3-b]pyridine (50g, 0.208mol) into the one-necked bottle, then add 100ml of chloroform, and then add dibromohydantoin (59.3g, 0.208mol) was he...
Embodiment 3
[0078] step one)
[0079] Add 5H-[1]-benzopyran[2,3-b]pyridine (50g, 0.27mol) into a one-necked flask, then add 500ml of dichloromethane, cool to 5°C, add anhydrous aluminum trichloride ( 180g, 1.35mol), and finally propionyl bromide (73.4g, 0.54mol) was slowly added dropwise, and the dropwise addition was completed in 30 minutes. During the dropwise addition, the temperature was controlled to be ≤10°C, and then the temperature was raised to room temperature to continue the reaction for 4 hours, and then the system was added to 250ml of ice water , adjust the pH to about 4 with sodium carbonate, filter, add 250ml of dichloromethane to the filtrate for extraction, add water to the organic layer after layering, and spin dry the organic layer to obtain 56g of a light red solid with a yield of 87%.
[0080] Step (2)
[0081] Add 7-(1-propionyl)-5H-[1]-benzopyran[2,3-b]pyridine (50g, 0.208mol) into a one-necked flask, then add 250ml of dichloromethane, and then add NBS ( 55g, 0.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com