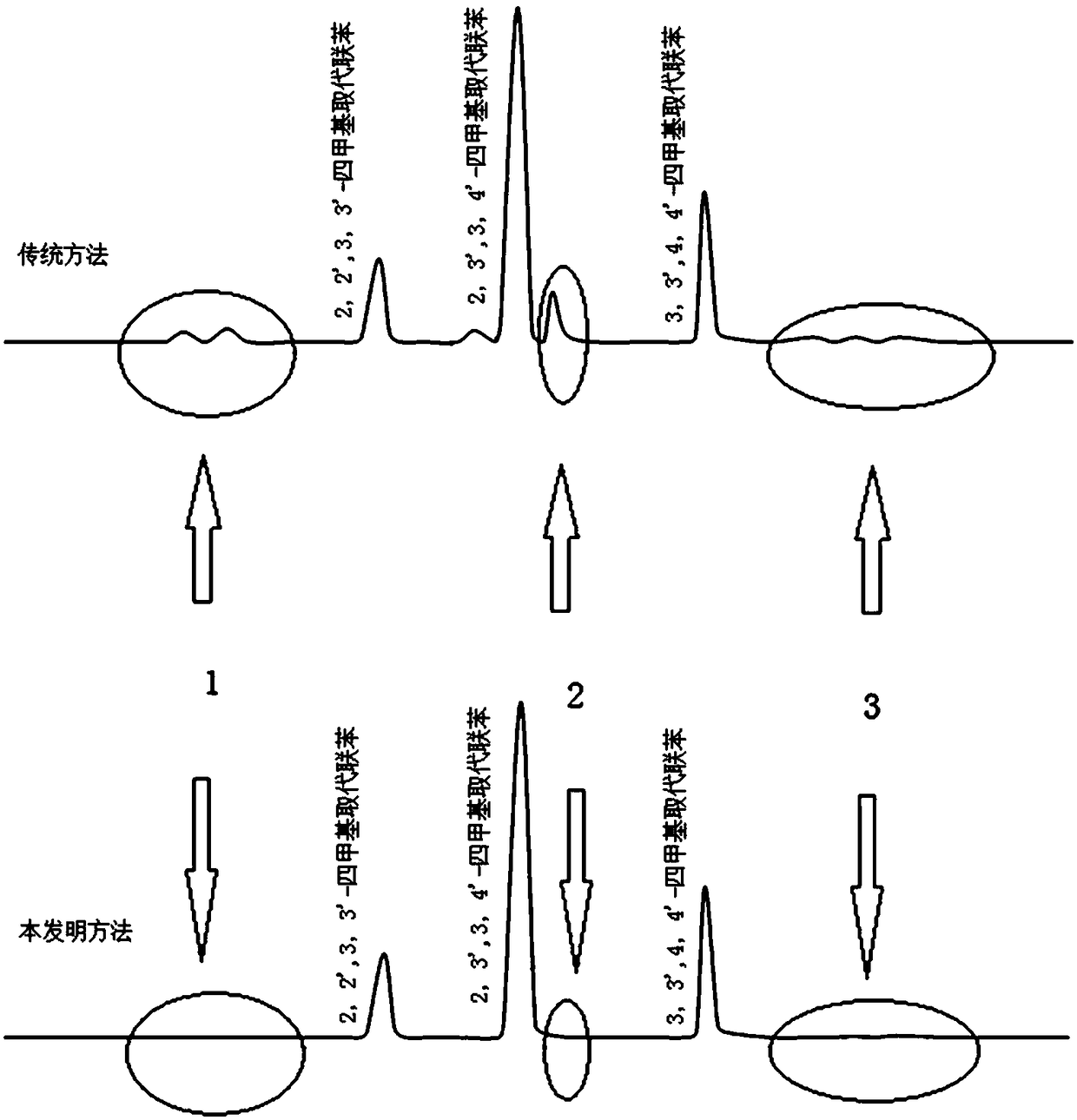
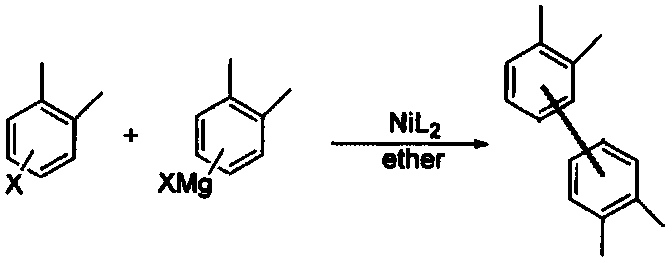
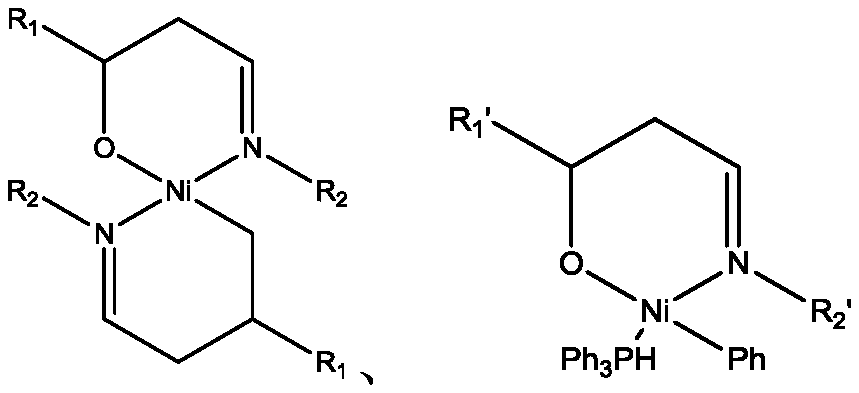
Preparation method of tetrahydrocarbyl substituted biphenyl
A technology of tetrahydrocarbyl and dihydrocarbyl benzene is applied in the field of polyimide monomer synthesis, which can solve the problems of high catalyst activity and excessive impurities in the product, so as to reduce the content of nickel salt, reduce the dosage of quencher, and delay catalysis effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation of 3,3',4,4'-Dimethyldiethyl-substituted biphenyl
[0047] (1) Preparation of catalyst
[0048] Carbonize polyacrylonitrile fibers with a crimp number of 34 at 482°C for 26 hours, and break them into particles with a particle size of 0.1-0.8 mm; carbonize polyacrylonitrile fibers, nickel acetylacetonate and DMF in a mass ratio of 3 : The ratio of 1:46 is evenly mixed and added to the pressurized reactor, start stirring, pass nitrogen replacement, and keep the pressure at 0.05MPa; heat the reactor to 70°C, soak for 7 hours, cool down, release the pressure, and filter A solid was obtained and dried at 175°C until the solvent evaporated completely. The catalyst is obtained by rolling.
[0049] (2) Preparation of Grignard reagent
[0050] The preparation of 4-chloro-substituted o-methylethylbenzene: get 1545g o-methylethylbenzene, 5g diphenyl sulfone, 5g anhydrous ferric trichloride, start stirring, and the rotating speed is at 100-200r / min, feed chlorine in ...
Embodiment 2
[0059] Preparation of 3,3',4,4'-tetramethyl-substituted biphenyl
[0060] (1) Preparation of catalyst
[0061] Carbonize aramid 1414 fibers with a crimp number of 66 at 684°C for 24 hours, and crush them into particles with a particle size of 0.1-0.8 mm; carbonize aramid 1414 fibers, nickel chloride and NMP at a mass ratio of 2.3 : The ratio of 1:7.67 is evenly mixed and added to the pressurized reactor, and the stirring is started, and nitrogen replacement is introduced, and the pressure is maintained at 1.8MPa; the reactor is heated to 184°C, and after immersion for 18 hours, the temperature is lowered, the pressure is released, and filtered A solid was obtained and dried at 230 °C until the solvent evaporated completely. The catalyst is obtained by rolling.
[0062] (2) Preparation of Grignard reagent:
[0063] Introduce high-purity nitrogen into a 1000ml four-neck flask with stirring, add 185g of 4-bromo-substituted o-xylene and 370g of highly anhydrous tetrahydrofuran,...
Embodiment 3
[0071] Preparation of mixed isomeric tetramethyl-substituted biphenyls
[0072] (1) Preparation of catalyst
[0073] Carbonize the trilobal polyester imide fiber with a crimp number of 34 at 545°C for 24 hours, and break it into particles with a particle size of 0.1-0.8mm; carbonize the trilobal polyester imide fiber , nickel bromide and DMAC are uniformly mixed according to the mass ratio of 1.9:1:26.1 and then added to the pressurized reactor, the stirring is started, nitrogen replacement is introduced, and the pressure is maintained at 0.4MPa; the reactor is heated to 80°C, impregnated After 12 hours, the temperature was lowered, the pressure was released, the solid was filtered out, and dried at 190° C. until the solvent evaporated completely. The catalyst is obtained by rolling.
[0074] (2) Preparation of Grignard reagent:
[0075] Pass into high-purity nitrogen in the 1000ml four-neck flask with stirring, add 140.5g of 3(4)-mixed chlorine-substituted o-xylene (the ma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com