Patents
Literature
110 results about "Sodium tert-butoxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
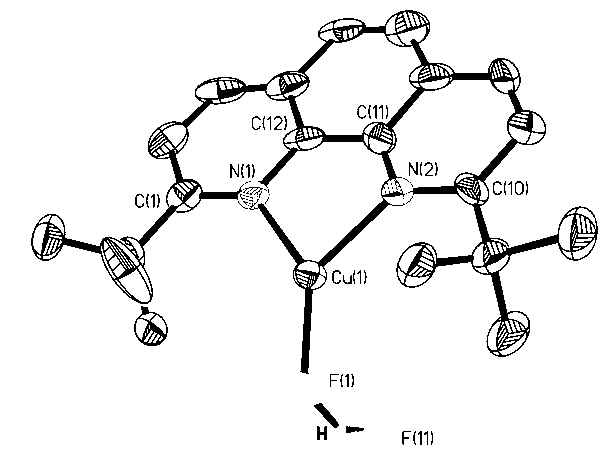
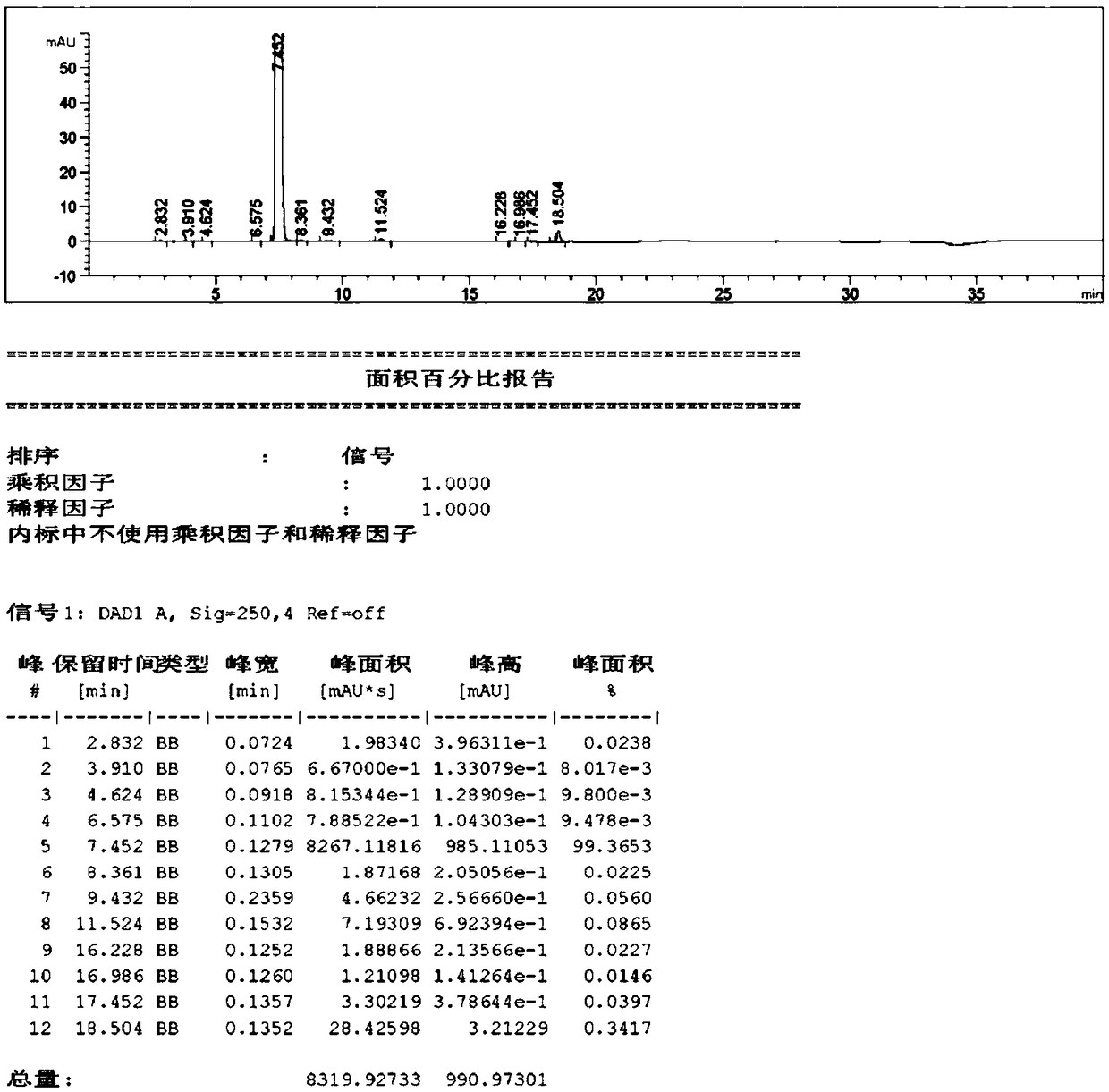
Sodium tert-butoxide is the chemical compound with the formula (CH₃)₃CONa. It is a strong base and a non-nucleophilic base. It is sometimes written in chemical literature as sodium t-butoxide. It is similar in reactivity to the more common potassium tert-butoxide.
Solid basic catalyst, preparation method of solid basic catalyst and application of solid basic catalyst in ester exchange reaction
InactiveCN102698811AImprove stabilityImprove catalytic performanceOrganic-compounds/hydrides/coordination-complexes catalystsPreparation from organic carbonatesOrganic baseSilicon oxide
The invention discloses a solid basic catalyst, a preparation method of the solid basic catalyst and an application of the solid basic catalyst in ester exchange reaction. The solid basic catalyst catalyzes the ester exchange reaction by the reaction of metal organic compound and hydroxyl on a carrier under the moderate conditions to synthesize dimethyl carbonate. The solid basic catalyst provided by the invention consists of metal organic alkali and the carrier. The metal organic alkali is linked to the carrier in bond-forming manner; the metal organic alkali is one or more of lithium methoxide, lithium ethoxide, lithium isopropoxide, lithium n-butoxide, lithium tert-butoxide, sodium methoxide, sodium ethoxide, sodium isopropoxide, sodium n-butoxide, sodium tert-butoxide, potassium methoxide, potassium ethoxide, potassium isopropoxide, potassium n-butoxide and potassium tert-butoxide; the carrier is one or more of silicon oxide, aluminium oxide, titanium oxide, zirconia, mesoporous silicon oxide synthesized by the template method, mesoporous aluminium oxide synthesized by the template method, mesoporous titanium oxide synthesized by the template method, and mesoporous zirconia synthesized by the template method.
Owner:NANJING UNIV OF TECH
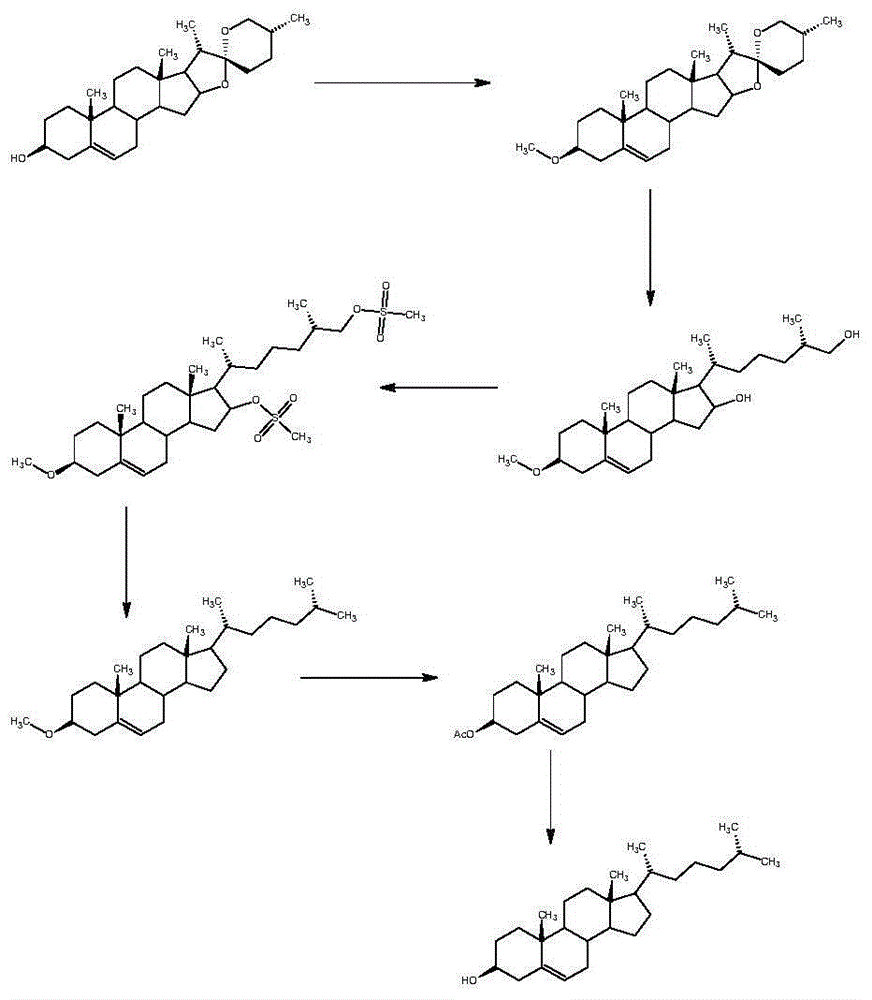
Synthetic method of cholesterol
InactiveCN104961788AReduce consumptionOmit ring opening reactionSteroidsCholesterolTriphenyl phosphonium
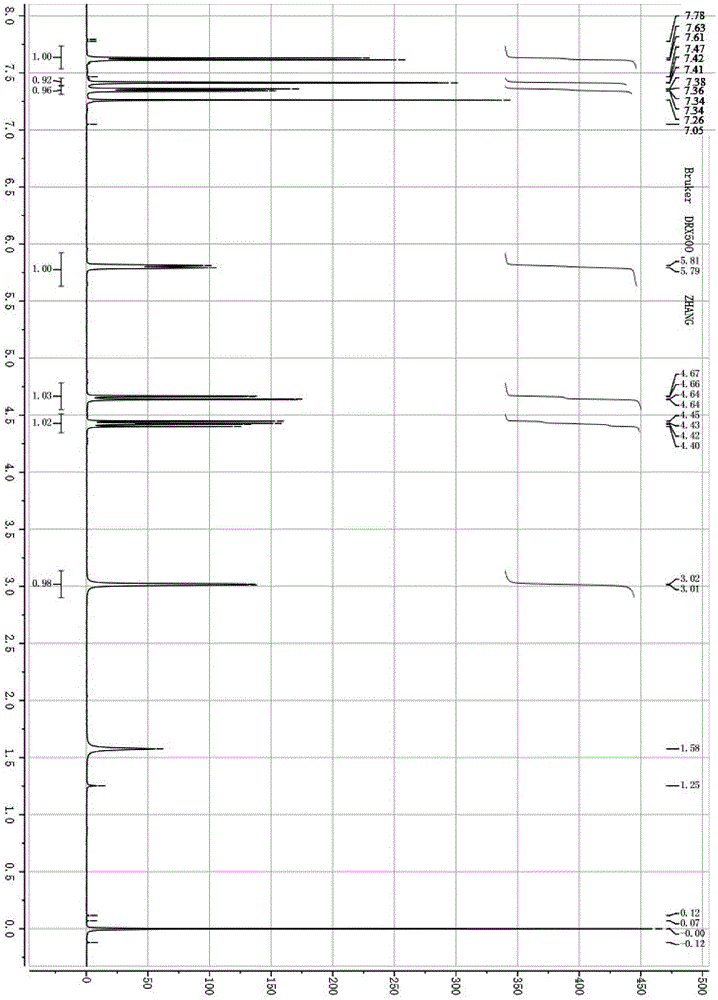
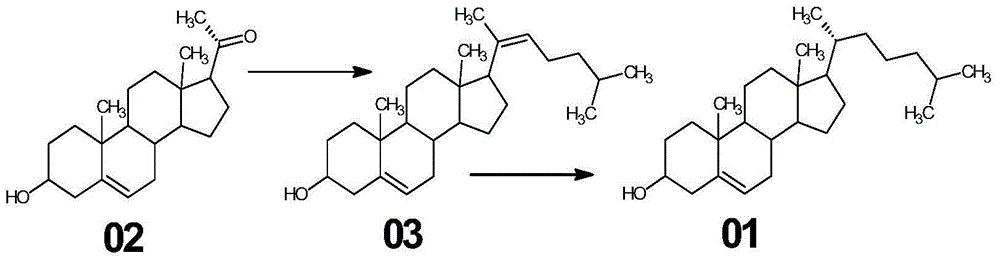
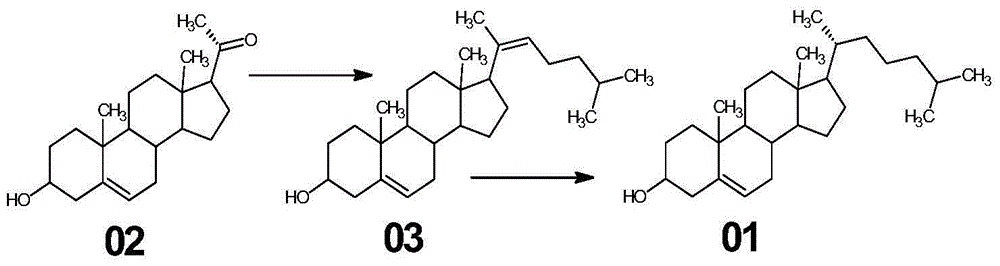
The invention relates to a synthetic method of cholesterol. The synthetic method of the cholesterol specifically comprises the following steps that 1, firstly, triphenylphosphine and 1-chlorine-4-methylpentane are used to react to obtain a 4-methyl pentyl triphenyl phosphonium chloride solution, then potassium tert-butoxide or sodium tert-butoxide is added to react to obtain a witting reagent, a witting reaction occurs by adding pregnenolone into the witting regent, and a compound 03 is obtained through extracting after complete reaction; 2, under the action of chirality phosphine ligand and a rhodium catalyst, an asymmetric hydrogenation reaction occurs to the compound to obtain the cholesterol. According to the synthetic method of the cholesterol, a 6-step reaction of an existing method is simplified into a 2-step reaction, and meanwhile the ring-opening reaction consuming a large amount of hydrochloric acid and zinc powder is omitted. The technology is simple, the consumption of raw materials is less, the yield is high, the cost is low, environmental protection is achieved, so that the economical effect and the environmental protection are achieved, and the industrial implementation is facilitated.
Owner:HUNAN KEREY BIOTECH
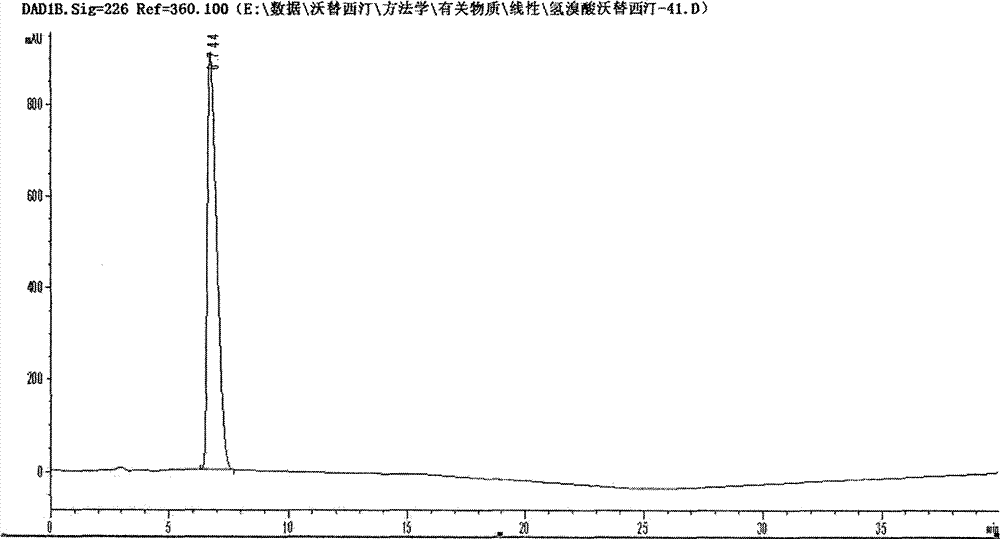
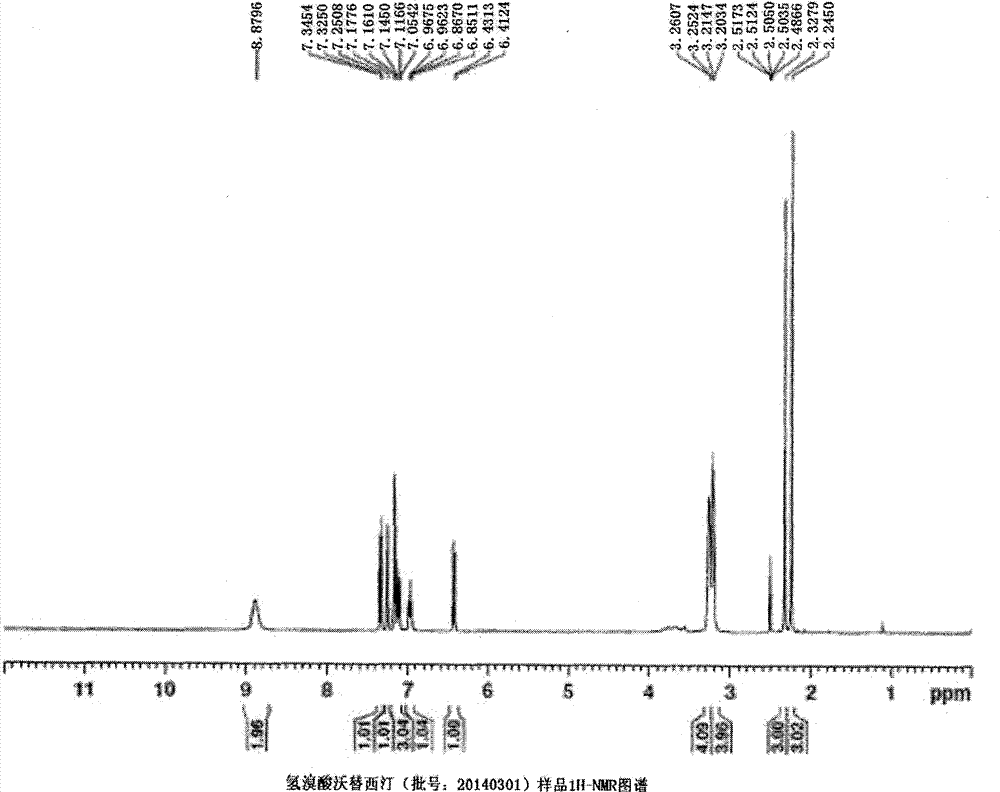
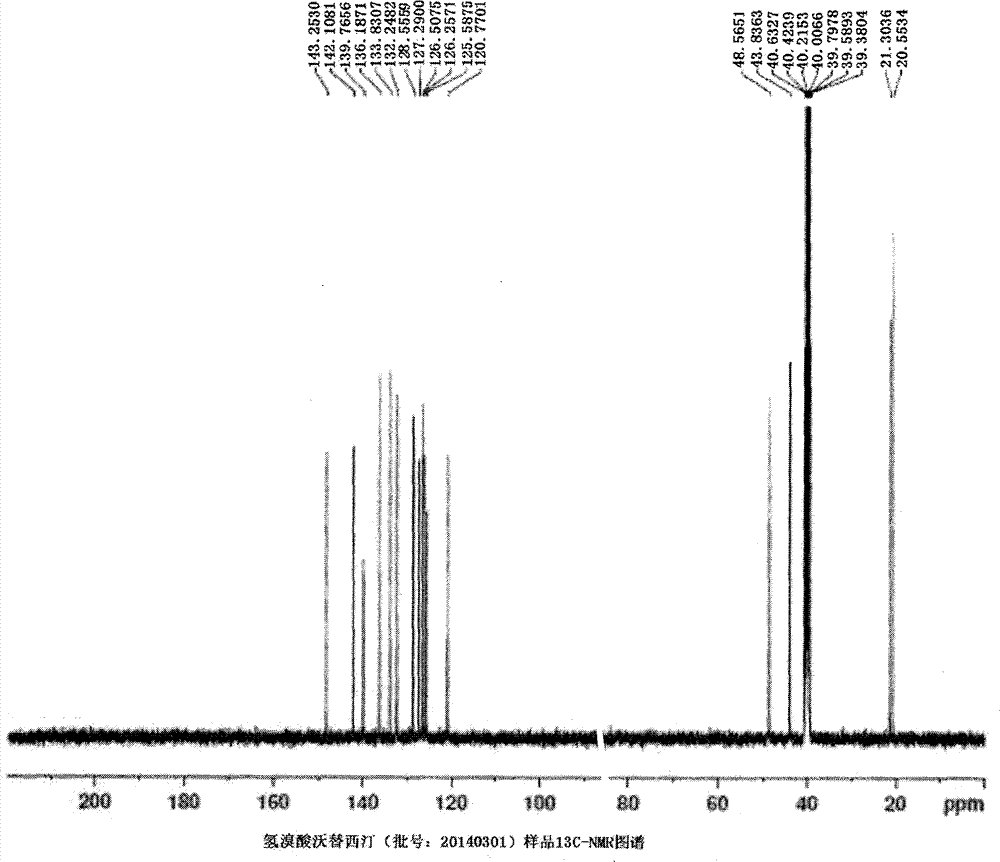
Novel preparation method of hydrobromic acid Vortioxetine beta crystalline form
The invention discloses a novel preparation method of a hydrobromic acid Vortioxetine beta crystalline form. The novel preparation method comprises the steps of firstly synthesizing 2-(2,4-dimethyl-phenylsulfanyl)chlorobenzene from 2-chlorobenzenethiol and 2,4-dimethylphenol, adding bi(dibenzylidene acetone)palladium, 1,1'-binaphthyl-2,2'-bisdiphenylphosphino, sodium tert-butoxide and toluene into a reaction bottle, mixing, adding other substances to prepare Vortioxetine, dissolving prepared Vortioxetine by virtue of ethyl acetate with the weight of 14-16 times of that of Vortioxetine to obtain rough hydrobromic acid Vortioxetine, and finally purifying rough hydrobromic acid Vortioxetine to obtain finished hydrobromic acid Vortioxetine. The preparation method has the beneficial effects that the raw materials are easily available, process reaction conditions are mild, a product is high in yield and purity, and industrial production is easily realized; prepared hydrobromic acid Vortioxetine is white crystalline powder, and the purity is more than 99.5%.
Owner:郑州大明药物科技有限公司
Efficient cyhalothrin
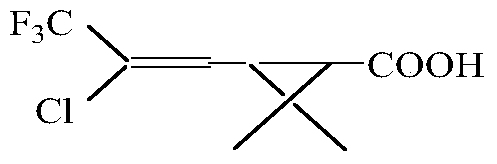
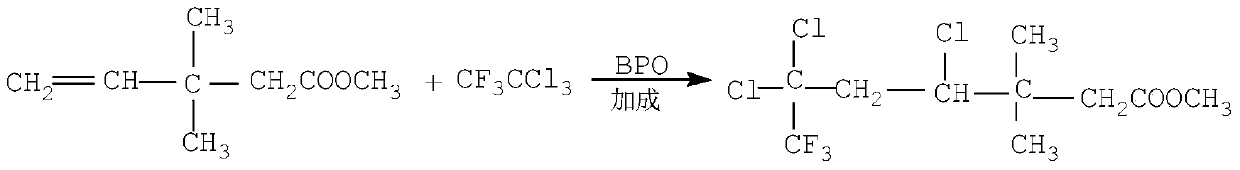
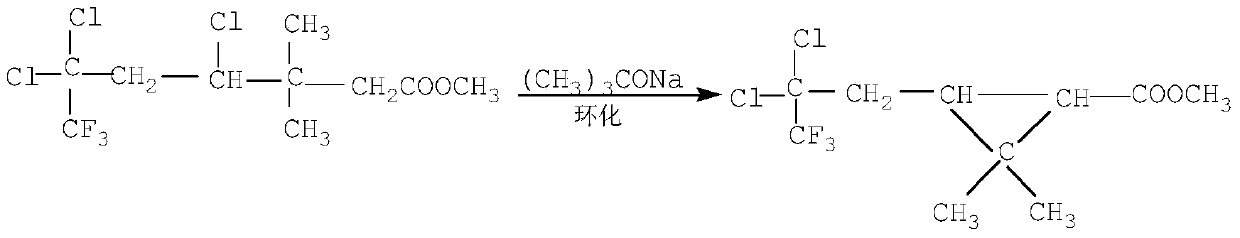
InactiveCN101434562AShort process routeImprove reaction efficiencyCarboxylic acid nitrile preparationOrganic compound preparation4-pentenoic acidFiltration
The invention relates to an efficient cyhalothrin and a manufacturing technology thereof, the manufacturing technology is as follows: (1) layer addition-reaction materials are 3, 3-dimethyl-4-pentenoic acid methyl ester and trichlorotrifluoroethane; (2) cyclization- reaction materials are tertiary butyl alcohol, Bifenthrin and sodium tert-butoxide; (3) elimination of hydrolysis-reaction materials are potassium hydroxide and cyclic Bifenthrin, and by exsolution, hydrolysis, separation and filtration 2-cis-3-(2-chloro-3, 3, 3-trifluro-1-propenyl)-2, 2-dimethyl cyclopropane carboxylic acid can be prepared; (4) chloroformylation-reaction materials are thionyl chloride, methylbenzene and 2-cis-3-(2-chloro-3, 3, 3-trifluro-1-propenyl)-2, 2-dimethyl cyclopropane carboxylic acid, and by exsolution and distillation, 3-(2-chloro-3, 3, 3-trifluoro-1-propenyl)-2, 2-dimethylcyclopropane carbonyl chloride is obtained; (5) esterification- reaction materials are phenylate aldehyde, sodium cyanide, PTC and 3-(2-chloro-3, 3, 3-trifluoro-1-propenyl)-2, 2-dimethylcyclopropane carbonyl chloride, by water rinse, exsolution and distillation, the cyhalothrin is prepared; and (7) epimerization-inoculating crystal is added for carrying out an epimerization reaction, and after the reaction, an end product-the efficient cyhalothrin is obtained by filtration and drying. As the invention adopts the technical proposal, the efficient cyhalothrin prepared by the invention greatly shortens the technological route and obviously improves the reaction efficiency through the improvement of technical skills, thereby reducing the cost of industrialization.
Owner:JIANGSU HUANGMA AGROCHEM
Copper fluoride (I) reagent as well as preparation method and application thereof
InactiveCN103254219AHigh reactivitySimple and fast operationCarboxylic acid nitrile preparationOrganic compound preparationHydrogen fluorideCopper fluoride
The invention discloses a copper fluoride (I) reagent as well as a preparation method and an application thereof. The copper fluoride (I) reagent is prepared through the following steps: stirring cuprous chloride and sodium tert-butoxide in a tetrahydrofuran solvent at room temperature to react, and adding a nitrogen ligand and triethylamine trihydrofluoride so as to obtain the copper fluoride (I) reagent which is stable in air. The triethylamine trihydrofluoride serves as a fluorine element source of the reagent, and the reagent can efficiently catalyze haloalkane to form hydrofluoroalkane. The high-purity and high-yield copper fluoride (I) reagent can be directly obtained through utilizing the industrial low-cost cuprous chloride, and has good reactivity with the hydrofluoroalkane containing a plurality of important functional groups; and a metal fluorinated reagent can be prepared without using toxic compounds and precious metals, the cost and operation requirements can be greatly lowered, and the copper fluoride (I) reagent has a good industrial application prospect.
Owner:FUZHOU UNIV
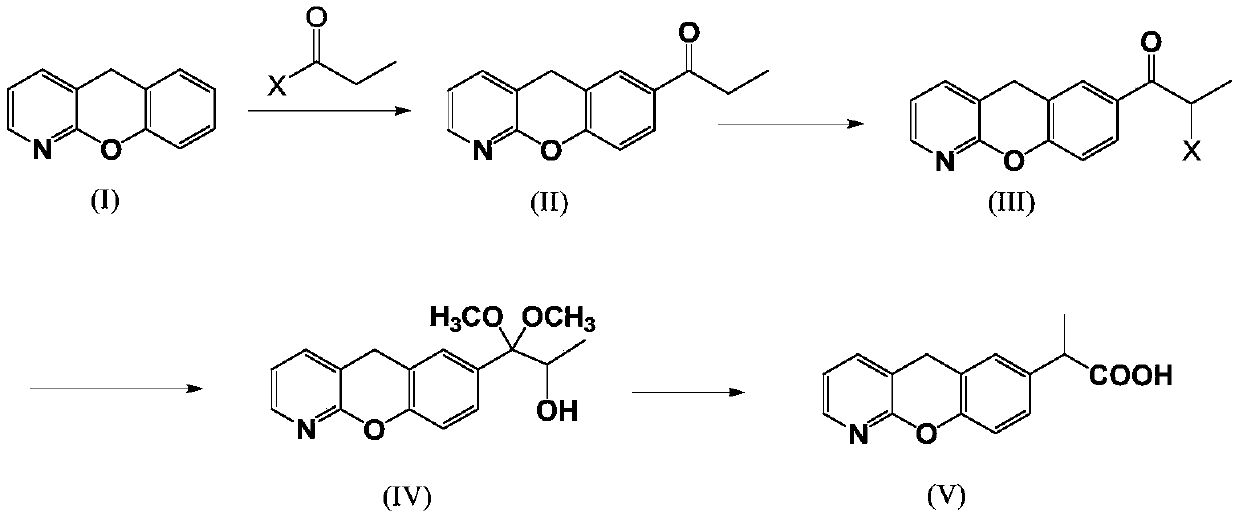
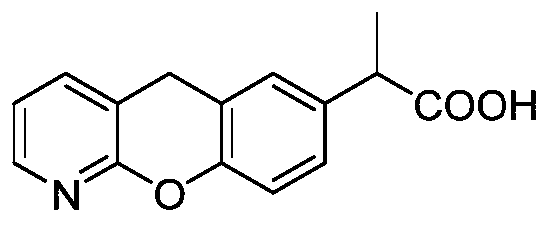
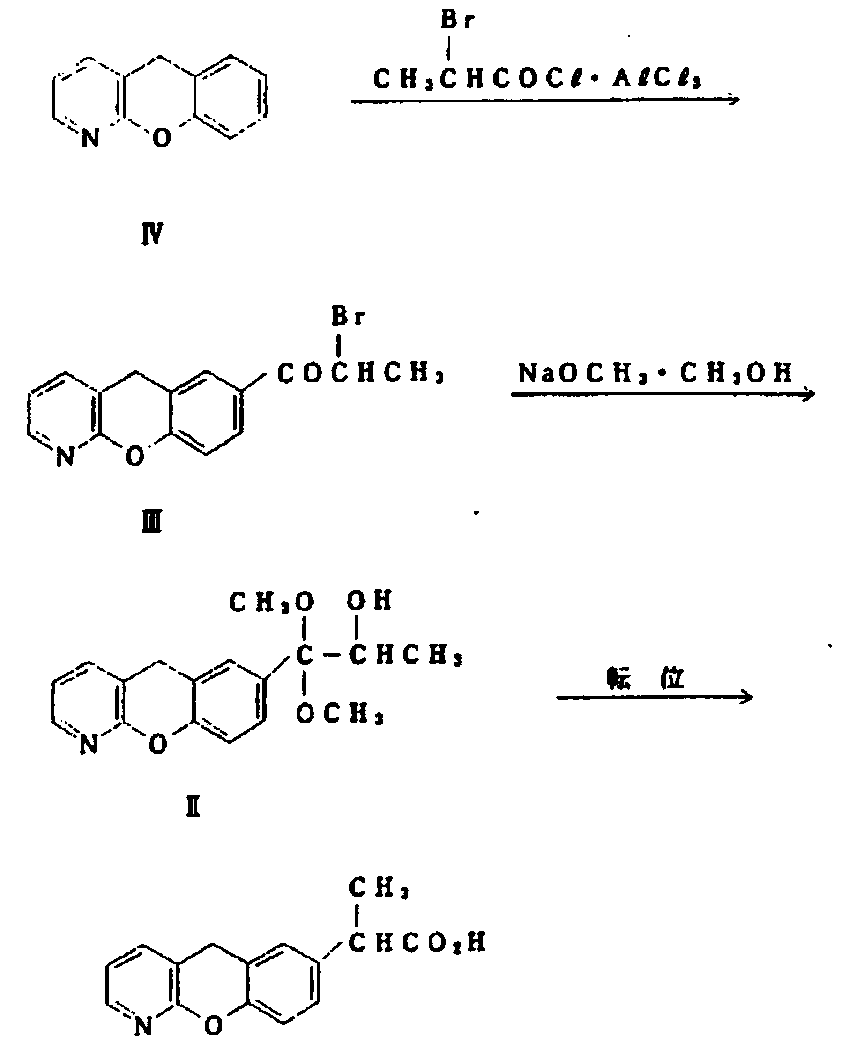
Novel preparation method of pranoprofen
The invention discloses a novel preparation method of pranoprofen. The novel preparation method of the pranoprofen has the advantages of being high in yield, having less impurities, and being safe andenvironmentally friendly. The novel preparation method of the pranoprofen comprises the following steps that step 1, 5H-[1]-benzopyran[2,3-b]pyridine and propionyl chloride are directly acylated; step 2, bromination is carried out; step 3, potassium tert-butoxide or sodium tert-butoxide is used for hydrolysis, and finally, rearrangement is carried out to obtain the pranoprofen.
Owner:GUANGDONG XIANQIANG PHARMA +2
Continuous production method for trifluoro monochloro chrysanthemic acid
InactiveCN105503582ABest production methodQuality improvementOxygen-containing compound preparationOrganic compound preparation4-pentenoic acidChrysanthemic acid
The invention discloses a continuous production method for trifluoro monochloro chrysanthemic acid. The method includes the steps of (1) performing an addition reaction to prepare 3,3-dimethyl-4,6,6-trichloro-7,7,7-trifluoro heptylic acid ester with 3,3-dimethyl-4-pentenoic acid methyl ester and trifluorotrichloroethane as initial raw materials; (2) performing a cyclization reaction with sodium tert-butoxide to generate cis(trans)-3-(2,2-dichloro-3,3,3-trifluoropropyl)-2,2-dimethyl cyclocarboxylate; and (3) performing a saponification reaction to prepare 3-(2-chloro-3,3,3-trifluoro-propylene-1-yl)-2,2-dimethylcyclopropane carboxylic acid, namely, the trifluoro monochloro chrysanthemic acid. In the invention, continuous control is employed in the production method, so that the method is simple in process, is high in equipment utilization, is high in yield and stable in quality of products, is low in production cost and has a wide application prospect.
Owner:LIANYUNGANG CCA CHEM CO LTD
Novel surface friction updating static mixing reactor
InactiveCN108607484AImprove reaction efficiencyHigh synthesis efficiencyFlow mixersTransportation and packagingNitrogenStatic mixer
The invention discloses a novel surface friction updating static mixing reactor which comprises a tert-butyl alcohol solution storage, a sodium tert-butoxide collector and a total mixer, wherein the tert-butyl alcohol solution storage is connected with the top of the total mixer through a liquid inlet pipe; a pre-outlet is opened in a bottom of the total mixer; the pre-outlet is connected with thesodium tert-butoxide collector by a material guiding pipe; a metal sodium block and nitrogen inlet is opened in a top side of the total mixer; a first mixing chamber is arranged below the metal sodium block and nitrogen inlet; a first SB type static mixer is arranged above the interior of the first mixing chamber; a first SX type static mixer is arranged at the lower part inside the first mixingchamber; a second mixing chamber is connected to the lower part of the first mixing chamber; a third mixing chamber is connected to the lower part of the second mixing chamber; a bottom chamber is connected to the lower part of the third mixing chamber; and a filter mesh is arranged in the bottom chamber. The novel surface friction updating static mixing reactor improves the synthesis efficiency of sodium tert-butoxide by combining different elements in the static mixer.
Owner:NANJING NORMAL UNIVERSITY
Preparation method of high-purity vortioxetine hydrobromide
ActiveCN104725335ARaw materials are easy to getProcess reaction conditions are mildOrganic chemistryChlorobenzene2-Chlorophenol
The invention discloses a preparation method of high-purity vortioxetine hydrobromide. The method comprises the following steps: firstly, synthesizing 2-(2,4-dimethyl phenyl sulfanyl) chlorobenzene from 2-chlorophenol and 2,4-dimethylbenzenethiol; then, adding di(dibenzylideneacetone)palladium, 1,1'-binaphthyl-2,2'-bis(diphenyl phosphine), sodium tert-butoxide, and methylbenzene into a reaction bottle to mix, and adding other materials so as to prepare vortioxetine; and dissolving the prepared vortioxetine by using 14-16 times of ethyl acetate, so that a vortioxetine hydrobromide coarse product is obtained; and finally, purifying the coarse product so as to obtain a vortioxetine hydrobromide fine-product. The method disclosed by the invention is easily-obtained in raw materials, mild in process reaction conditions, high in product yield, high in product purity, and convenient for industrial production. Prepared vortioxetine hydrobromide is white crystalline powder, and the purity is more than 99.5%.
Owner:郑州大明药物科技有限公司
Preparation method of selexipag
ActiveCN106008364AReasonable designHigh reactivityOrganic chemistryPotassium tert-butoxideOrganic solvent
The invention discloses a preparation method of selexipag, and relates to the technical field of preparation of drugs for treating pulmonary hypertension. The preparation method comprises the following steps: adding a compound as shown in the formula I and a compound as shown in the formula II in an organic solvent; adding alkali; stirring at the reaction temperature of 10-40 DEG C and carrying out Williamson reaction; synthesizing a compound as shown in the formula A, namely the selexipag. The organic solvent is 1-4-dioxane; the alkali is sodium tert-butoxide, potassium tert-butoxide or mixed alkalis of sodium tert-butoxide and potassium tert-butoxide. A synthetic route is reasonable in design, reaction conditions are mild, the cost is low, special equipment is not required, a product is high in purity and high in yield, and aftertreatment is simple and convenient, so that the preparation method of the selexipag is suitable for industrial production.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
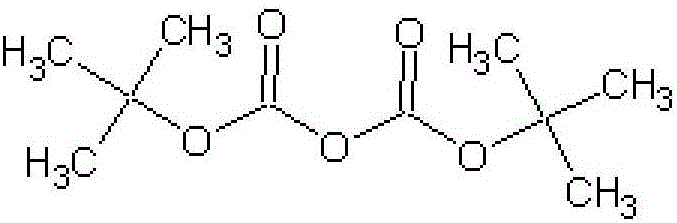
Preparation method of di-tert-butyl dicarbonate
The invention relates to a preparation method of di-tert-butyl dicarbonate and belongs to the technical field of synthesis of pharmaceutical intermediates. The preparation method comprises the following steps: adding metal sodium into xylol; heating to obtain sodium sand; then dropwise adding tert-butyl alcohol and carrying out pumping filtration to obtain sodium tert-butoxide; dissolving the sodium tert-butoxide into petroleum ether; introducing carbon dioxide and reacting to obtain a monoester sodium salt solution; adding a catalyst and slowly dropwise adding diphosgene to react; after reacting, standing and carrying out the pumping filtration; and washing with water, drying, distilling, cooling and crystallizing to obtain the di-tert-butyl dicarbonate. According to the preparation method, the sodium tert-butoxide is prepared from the metal sodium and the di-tert-butyl dicarbonate is prepared from the sodium tert-butoxide; a pumping filtration method is used for replacing a previous distillation method, so that the process is simpler and more energy is saved; the petroleum ether is used for replacing n-hexane and toluene, so that the production cost is reduced and a product is easier to purify; and finally, after the reaction, the pumping filtration is carried out and then water washing is carried out, so that the amount of wastewater is reduced and the environment-friendly treatment cost is reduced.
Owner:SHANDONG JINCHENG KERUI CHEMICAL CO LTD
Photoelectric material and preparation method thereof and application to organic electronic device
ActiveCN105669977AOverlap smallEasy to prepareSolid-state devicesSemiconductor/solid-state device manufacturingFluorescenceSide chain
The invention discloses a photoelectric material and a preparation method thereof and application to an organic electronic device. The preparation method of the photoelectric material includes the steps that a halogenated compound of M1 or M2 is prepared firstly, wherein M1 or M2 monomers serve as raw materials, N-bromosuccinimide or liquid bromine is added, in an organic solvent of concentrated sulfuric acid or hydrogen peroxide, a reaction is conducted under a normal-temperature or heating and stirring conditions, purification is conducted, and the halogenated compound of M1 or M2 is obtained; then secondary amine is prepared through a C-N coupled reaction, wherein Pd2(dba)3 and DPPF are firstly added to a container, after stirring in a benzene series solvent, the halogenated compound of M1 or M2 is added, primary amine of Ar1 or Ar4 and sodium tert-butoxide are added, and heating reflux is conducted; finally the photoelectric material is prepared. A D-A-D-A type non-conjugate polymer is synthesized through a C-N coupling polymerization method, in addition, a side chain with an electron-donating group is arranged, LUMO and HOMO electron clouds are slightly overlapped, and therefore the characteristic of thermotropic delayed fluorescence can be easily achieved.
Owner:东莞伏安光电科技有限公司
Method for preparing di-tert-butyl dicarbonate
ActiveCN102557952AHigh yield of catalytic reactionMild reaction conditionsProductsReagentsSatiationsCarbonyl chloride
The invention discloses a method for preparing di-tert-butyl dicarbonate. The method comprises the steps of: adding sodium tert-butoxide and normal hexane in a reaction kettle, heating until the sodium tert-butoxide and the normal hexane resolve completely under the protection of N2, stirring and cooling to 0 to 50 DEG C; after introducing carbon dioxide into the reaction kettle until satiation, adding a three way catalyst into the liquid reactant; controlling the internal temperature of the reaction kettle to a range from minus 12 DEG C to 20 DEG C; adding solid carbonyl chloride into the reaction kettle for 10 times every 0.5 hour, and reacting for 3 hours after adding is finished; after reacting, adding water and washing, and vaporizing the normal hexane solvent off under normal pressure to obtain a crude product of di-tert-butyl dicarbonate; and crystallizing at the temperature of below 0 DEG C, and centrifugalizing the mother liquid after crystallizing is finished completely so as to obtain the required di-tert-butyl dicarbonate.
Owner:GENCHEM & GENPHARM CHANGZHOU CO LTD
Method for synthesizing polyimino-alcohol
InactiveCN101186680AImprove heat resistanceExcellent dynamic mechanical propertiesSynthesis methodsKetone
The invention relates to a synthesis method of polyuralcohol, belonging to polymer technical field, which comprises that adds dihalide aromatic ketone, sodium tert-butoxide NaOt-Bu, 1, 1'-dinaphthalene-2, 2'-diphenylphosphino BINAP, aromatic diamine and Pd2(dba)3 into a three-mouth flask with nitrogen gas feeding in turn, wherein the mole ratio is 1:1:2.8:0.03:0.01, adds N, N-dimethylacetamide until the monomer density is 0.5-0.65mol / L, increases temperature from 20DEG C to 100DEG C, keeps for 4-6h, increases temperature from 100DEG C to 150-200DEG C, keeps for 20-24h, cools to 20DEG C, adds the mixture of ammonia and methanol at the ratio of 1:4(L / L), mixes, stands for 1h, washes via the mixture of ammonia and methanol for three times, filters, dries at vacuum for 6h, mixes and dissolves poly(imino ketone) and benzoyl chloride in tetrahydrofuran for 2-12h at 1-3DEG C, to obtain polyuralcohol to be dried. The synthesized polyuralcohol Tg is higher than 190DEG C and TD is higher than 450DEG C, with better heat stability and dynamic mechanical stability.
Owner:QINGDAO UNIV OF SCI & TECH
Preparation method of 2-trifluoromethyl indole derivatives
InactiveCN101987832AOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsNitrogenSolvent
The invention belongs to a preparation method of 2-trifluoromethyl indole derivatives. The method of the invention comprises the following steps: enabling F-113a serving as a fluorine raw material to react with o-chlorobenzaldehyde or o-bromobenzaldehyde to prepare alkenyl polyhalide, than reacting with any primary amine to selectively synthesize a series of 2- trifluoromethyl indole derivatives, and finally synthesizing a series of sertindole derivatives by structural modification. The reaction is characterized in that the Pd2(dba)3 is taken as a catalyst, sodium tert-butoxide is taken as alkali under participation of nitrogen and phosphorus ligands, and toluene as a solvent. The method has the advantages of short synthetic route, short reaction time, good reaction selectivity, good yield and the like, and is simple in operation.
Owner:WENZHOU UNIVERSITY
Synthetic method of di-tert-butyl dicarbonate
The invention relates to a synthetic method of di-tert-butyl dicarbonate, and belongs to the technical field of synthesis and purification of chemical engineering products. The synthetic method comprises the following steps: adding tert-butanol, n-hexane and metal sodium to an autoclave under the protection of nitrogen to obtain sodium tert-butoxide, introducing CO2, stirring the sodium tert-butoxide, adding a catalyst, dropwise adding triphosgene dissolved in n-hexane to the autoclave, carrying out a heat insulation reaction, carrying out reduced pressure distillation to remove the n-hexane, adding water, washing the obtained reaction product, layering the washed reaction product, taking the above obtained organic phase, and carrying out cooling, freeze crystallization, centrifuge and drying to obtain the di-tert-butyl dicarbonate. The di-tert-butyl dicarbonate is prepared from the sodium tert-butoxide, a single solvent is used, experiment steps are simplified, normal pressure solvent removal is carried out after the reaction, and water washing is carried out, so the use amount of a dehydrating agent is reduced, water in the solvent is controlled, and the solvent recovery treatment is convenient; and triphosgene is highly-efficiently and easily degraded, and the catalyst is cheap and is easy to obtain, so the production cost is reduced, and the reaction efficiency is improved.
Owner:SHANDONG JINCHENG KERUI CHEMICAL CO LTD
Preparation method of Venetoclax intermediate and product
InactiveCN109320516AModerate process parametersMeet the process parameter requirementsOrganic chemistryBulk chemical productionReaction temperaturePotassium
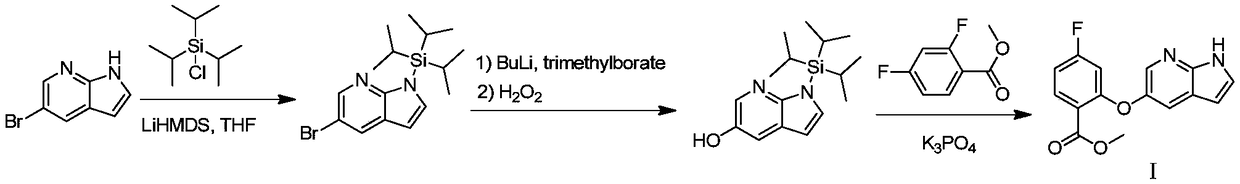
The invention discloses a preparation method of a Venetoclax intermediate. 5-bromine-7-azaindole (II) is used as a raw material; an upper protecting group generates 5-bromine-1-(Triisopropyl silicyl)-1H-pyrrolo[2,3-b]pyridine (III); a compound (III) and Triisopropyl borate react and then are oxidized to obtain 1-(Triisopropyl silicyl)-1H-pyrrolo[2,3-b]pyridine-5-alcohol (IV); and a compounds (IV)and 2,4-difluoro methylbenzoate react to generate 2-(1H-pyrrolo[2,3-b]pyridine-5-yloxy)-4-methyl 2-fluorobenzoate (I). According to the preparation method, lithium bisamide is replaced with tertiary butanol sodium / potassium, thus the production cost is lowered, trimethyl borate is replaced with the Triisopropyl borate, equipment input is less in the synthesis process, and the operation flow is simplified; and most of all, reacting under the ultra-low temperature is not needed, the reaction temperature can be minus 20-minus 10 DEG C, general equipment can all meet the requirements, energy consumption is lowered, operation is convenient, and the preparation method is suitable for industrial production.
Owner:CHONGQING SANSHENG IND CO LTD
Method of preparing granular sodium tert-butoxide
ActiveCN102001914AAvoid health hazardsAvoid harmPreparation of metal alcoholatesOrganic solventNitrogen gas
The invention discloses a method of preparing granular sodium tert-butoxide, which includes the following steps: using metal sodium, tert-butanol and an inert organic solvent as raw materials; adding the tert-butanol and the inert solvent into a reaction kettle and then adding the metal sodium into the reaction kettle; under the protection of a nitrogen gas, refluxing for 8-40 h; at a normal temperature, distilling the tert-butnaol; after totally distilling the tert-butanol, reducing the pressure to -0.08 to -0.098 MPa to distil the remained insert organic solvent; then adjusting the stirringrate of the reaction kettle to 10-80 r / min; reducing the pressure to distil the inert organic solvent; obtaining a residual granular sodium tert-butoxide product in the reaction kettle; cooling the product to less than 40 DEG C to obtain the required granular sodium tert-butoxide. The method can perform a production by using universal chemical equipment. An operation condition is moderate; a protection technique is simple; and the raw materials used are easily obtained. The quality of the product is stable and reliable. The shelf life of the product can be effectively prolonged.
Owner:GENCHEM & GENPHARM CHANGZHOU CO LTD
Preparation method for ditertbutyl dicarbonate
ActiveCN102351708ASimple processHigh yieldPreparation from carbon dioxide or inorganic carbonatesReaction temperatureToluene
The invention discloses a preparation method for ditertbutyl dicarbonate. In the method, the ditertbutyl dicarbonate is prepared from self-made sodium tert-butoxide solution, diphosgene and a catalyst under a certain condition. The preparation method has the advantages of simple process, high yield, high purity, capability of raising reaction temperature by substituting normal hexane with toluene(normal hexane is used in the conventional method) and higher safety.
Owner:THE IT ELECTRONICS ELEVENTH DESIGN & RES INST SCI & TECHNOLOGICAL ENG
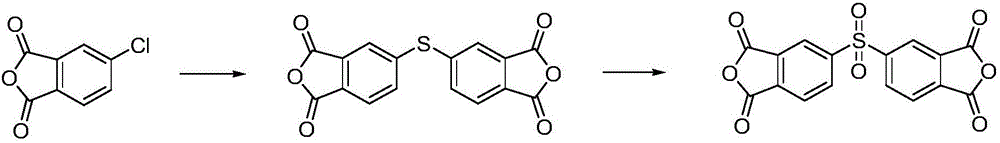
Preparation method of 3,3',4,4'-diphenyl sulfone tetracarboxylicdianhydride
The invention discloses a preparation method of 3,3',4,4'-diphenyl sulfone tetracarboxylicdianhydride. The preparation method comprises the following steps that 1, 4-chlorophthalic anhydride is dissolved into dimethyl sulfoxide, then sulfur and sodium tert-butoxide are added, carbon disulfide is dropwise added when the temperature is increased to 40 DEG C, after the raw materials completely react, part of the solvent is recycled under reduced pressure, a remaining product is added into water, and filtering, water washing and drying are conducted to obtain 3,3',4,4'-diphenyl thioether tetracarboxylicdianhydride; 2, 3,3',4,4'-diphenyl thioether tetracarboxylicdianhydride is dissolved into acetonitrile, ceric ammonium nitrate, tetrabutylammonium iodide and water are added, potassium persulfate is added in batches under the heating condition, after 3,3',4,4'-diphenyl thioether tetracarboxylicdianhydride completely reacts, water is added into reaction liquid, the temperature is lowered to 5 DEG C, filtering, water washing and drying are conducted, and 3,3',4,4'-diphenyl sulfone tetracarboxylicdianhydride is obtained. The preparation method of 3,3',4,4'-diphenyl sulfone tetracarboxylicdianhydride has the following advantages that the raw materials are easy to purchase, the reaction solvent can be recycled, aftertreatment operation is easy, the yield is high, the product quality is good, and environmental pollution is low.
Owner:天津众泰材料科技有限公司
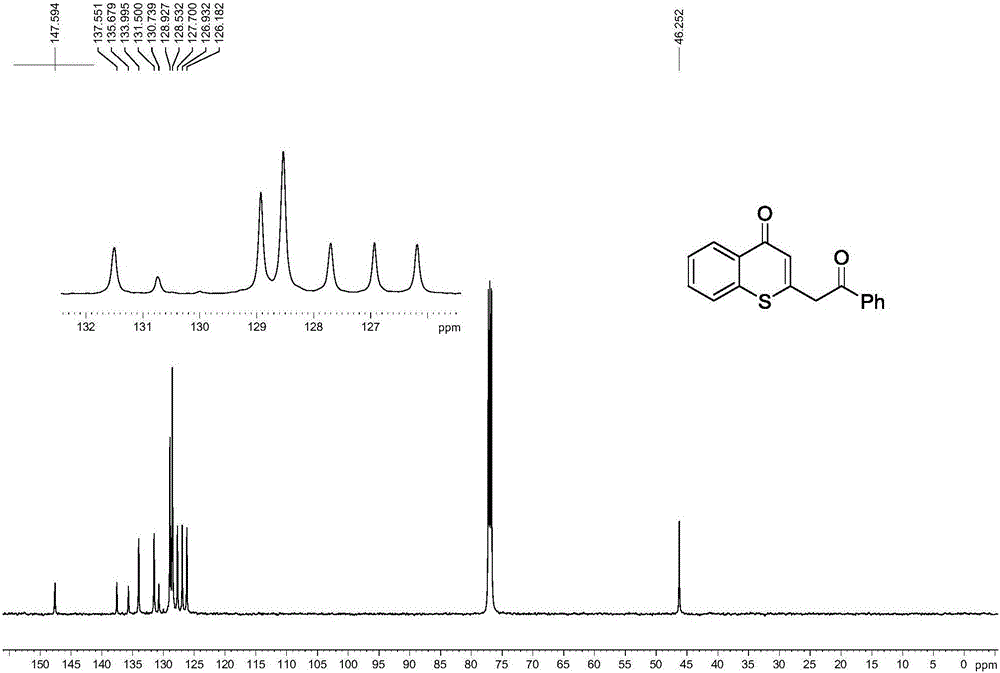
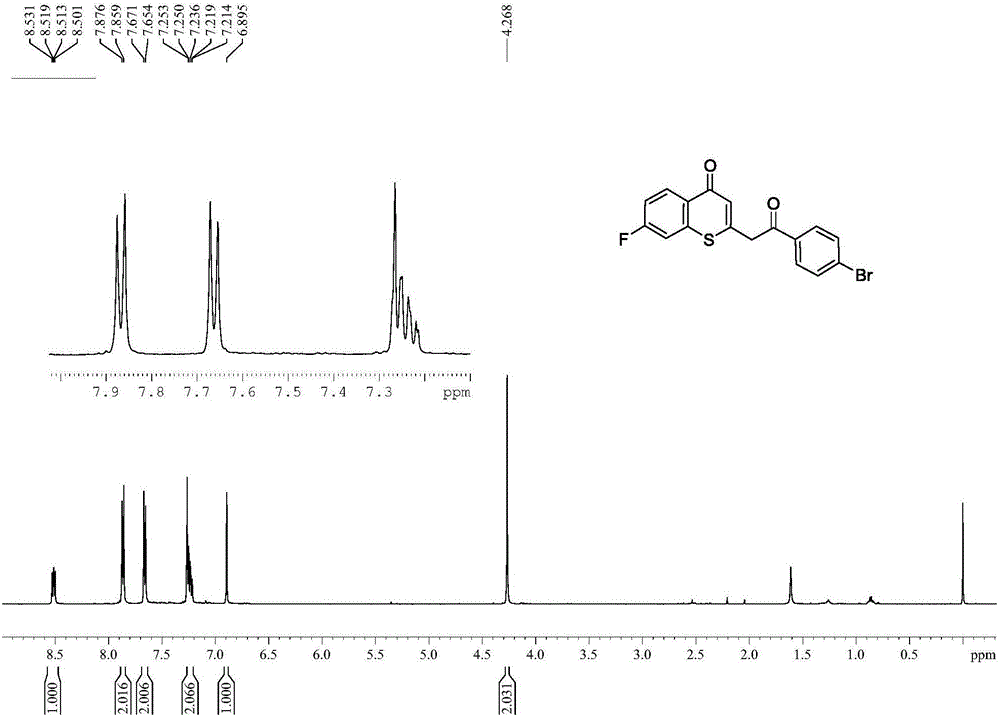
Preparation method of multi-substituted thiochromanone derivative
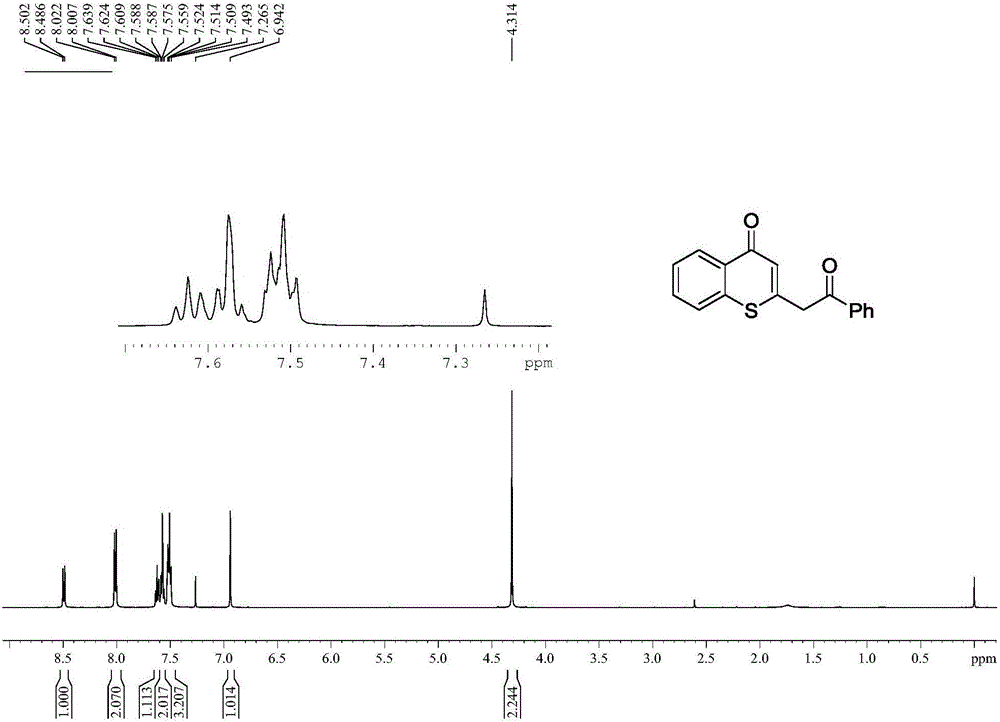
InactiveCN106083810AThe synthesis method is scientific and reasonableThe synthesis method is simpleOrganic chemistryRotary evaporatorOrganic synthesis
The invention discloses a preparation method of a multi-substituted thiochromanone derivative, and belongs to the technical field of organic synthesis. The method comprises the steps of: adding beta-carbonyl dithiocarbonic acid, substituted o-bromoacetophenone, copper iodide, phenanthroline and sodium tert-butoxide to a reactor, adding toluene as a solvent, and heating to the end of the reaction under the protection of nitrogen; after cooling, adding hydrochloric acid to adjust the pH into a neutral state; extracting three times with ethyl acetate, combining the organic phase, adding magnesium sulfate, drying, filtering, concentrating the filtrate in a rotary evaporator to obtain a crude product, and isolating by column chromatography silica gel to obtain the product. The method synthesis method of the multi-substituted thiochromanone derivative provided by the invention is scientific, reasonable, and simple; and the product is easy to be purified. The reaction equation is as follows.
Owner:QINGDAO UNIV OF SCI & TECH
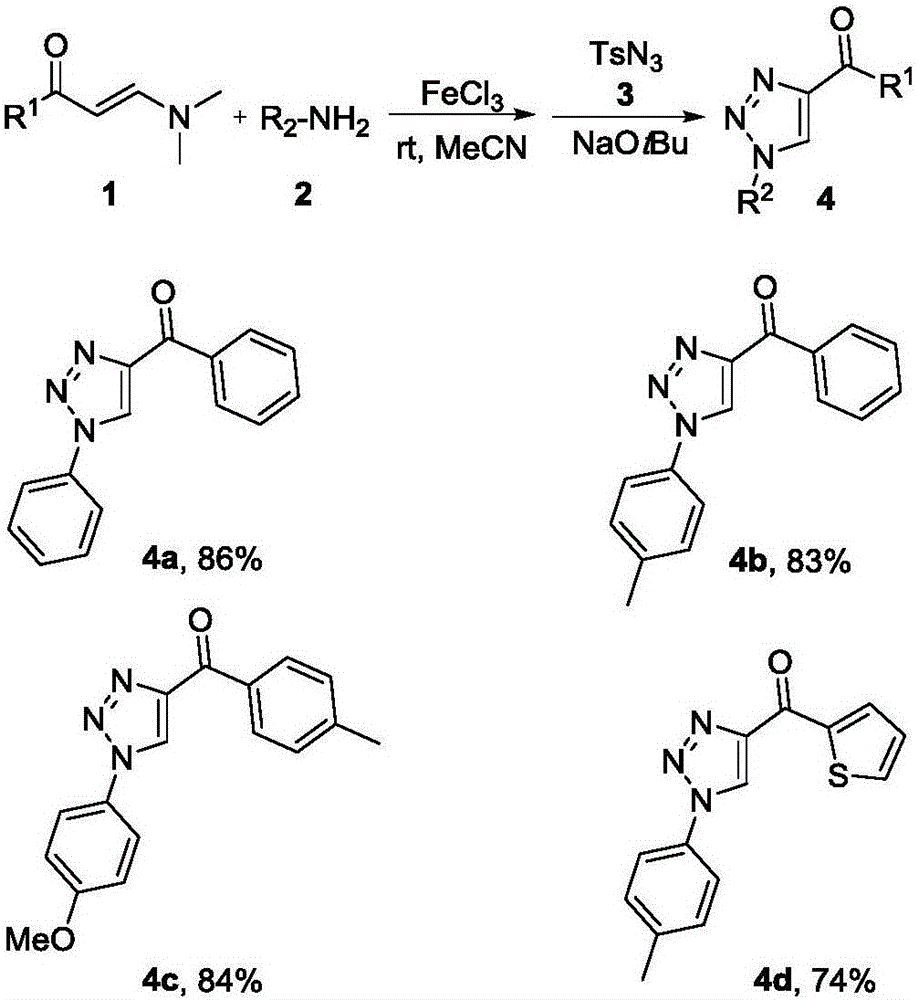
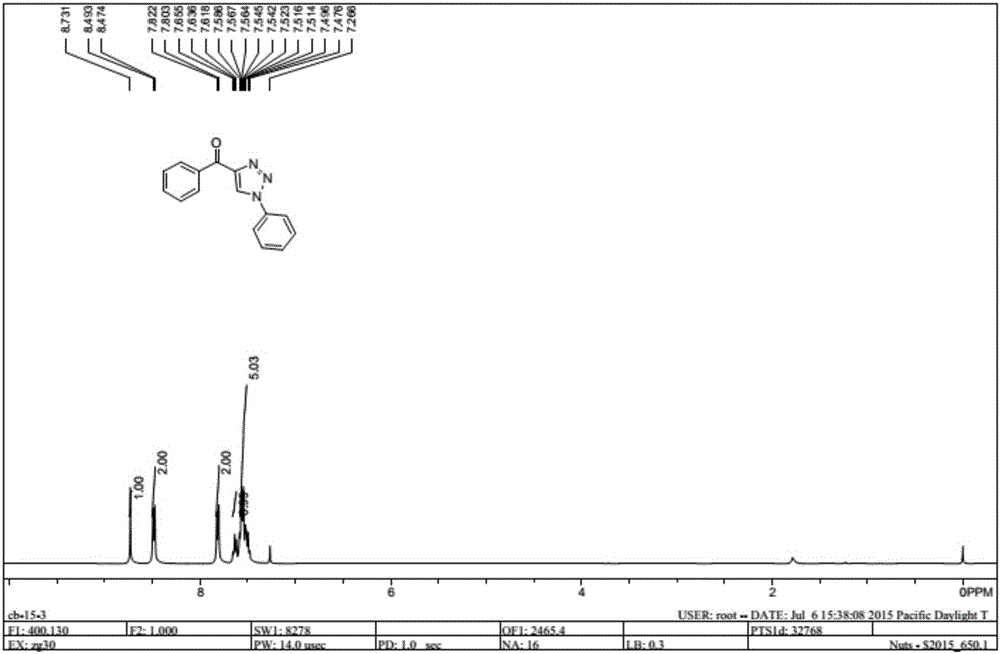
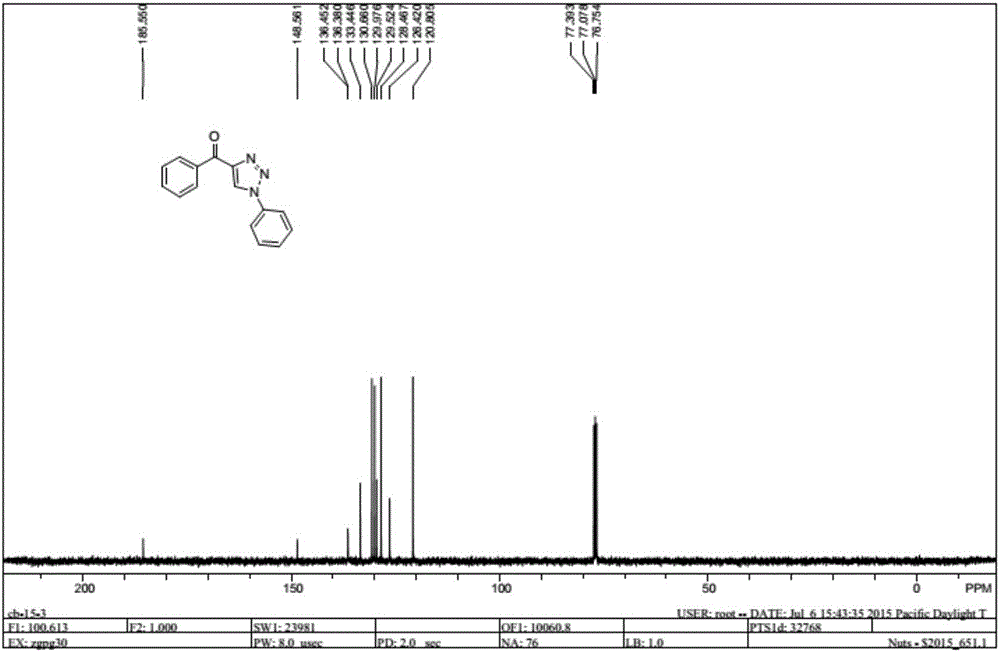
Method for synthesizing 1, 4-bis-substituted-1, 2, 3-triazole by cycloaddition reaction of base catalysis enaminone and sulfonyl azide
ActiveCN106187922ARaw materials are cheap and easy to getSynthetic conditions are mildOrganic chemistryCycloadditionSilica gel
The invention discloses a method for synthesizing 1, 2, 3-triazole by cycloaddition reaction of base catalysis enaminone and azide. According to the method, propargyl esters, primary amine and sulfonyl azide serve as raw materials, the enaminone with an NH structure is generated in situ, the raw materials are stirred at room temperature for two hours under catalysis of secondary amine and sodium tert-butoxide, 1, 4-bis-substituted-1, 2, 3-triazole compounds can be obtained with high yield, and products are subjected to chromatographic purification by a silica gel column. The method has the advantages that reaction conditions are mild, reaction can be smoothly realized at the room temperature, reaction time is short, two steps can be finished totally for four hours, the raw materials are green and low in cost, ferric chloride used in first-step operation is an environment-friendly catalyst, metal is omitted in second-step reaction, the method is greener than most methods for synthesizing similar compounds, and the method is simple in overall operation and suitable for large-scale production.
Owner:JIANGXI NORMAL UNIV
Method for preparing compound 2-(imidazo [1, 2-a] pyridine-3-yl) acetonitrile
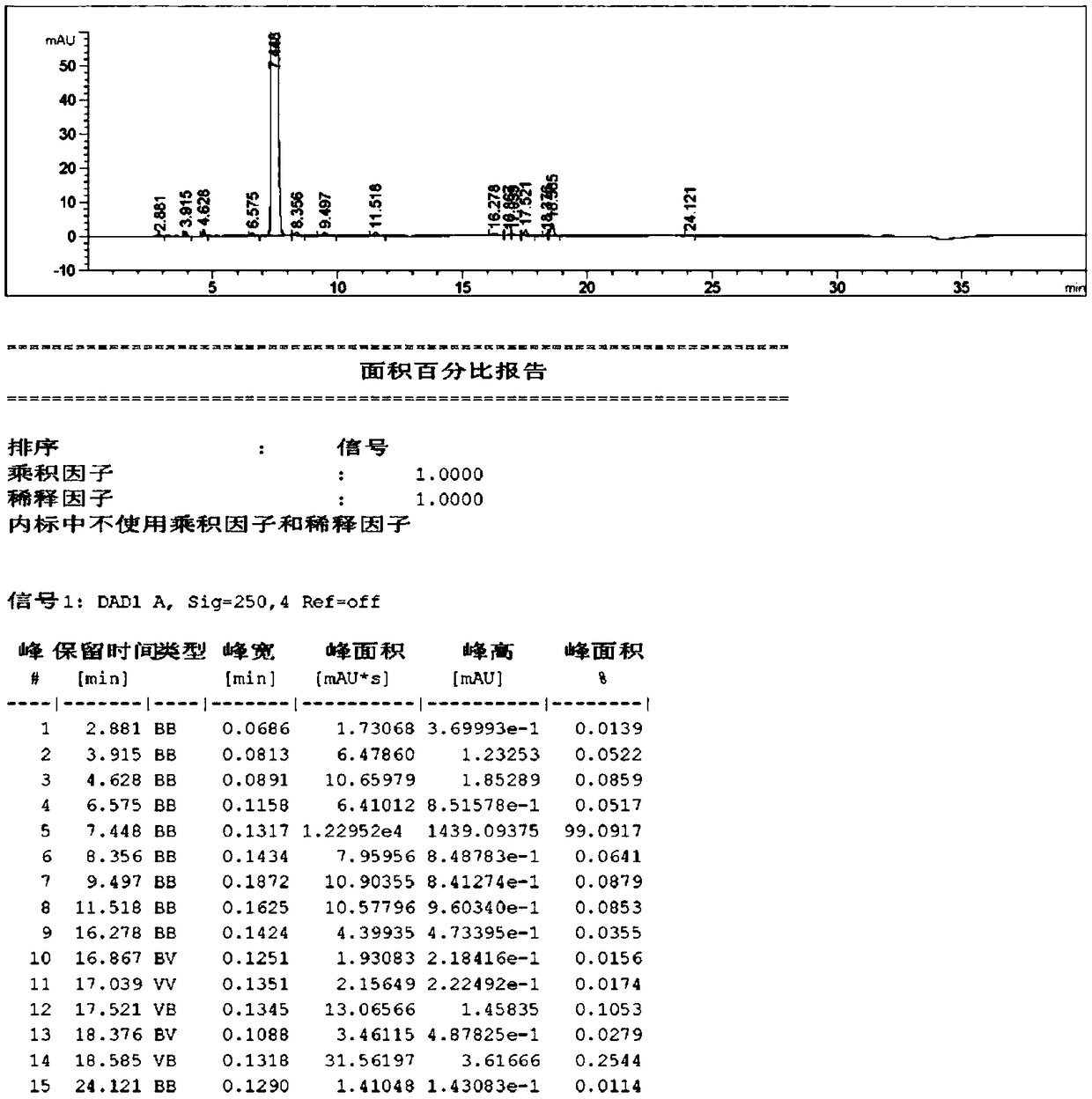
The invention discloses a method for preparing a compound 2-(imidazo [1,2-a] pyridine-3-yl) acetonitrile, which is a new method for preparing a key intermediate of minodronic acid, comprising the steps of: with a compound 3- imidazo [1, 2-a] pyridine carboxaldehyde with the formula (II) and a compound tosylmethyl isocyanide with the formula (III) as raw materials, carrying out chemical reaction in an organic solvent containing glycol dimethyl ether and an alkaline medium of potassium tert-butoxide or sodium tert-butoxide; separating and purifying to obtain the compound 2-(imidazo [1, 2-a] pyridine-3-base) acetonitrile, wherein the process of the chemical reaction is disclosed in the specification of the invention. In the invention, by means of the method for preparing the compound with the formula (I), the reaction procedure is shortened, the technical process of the whole reaction is simplified, the use of a highly toxic material sodium cyanide is avoided, the operability of the reaction is strong, the yield is high, and the obtained product has high purity, lower cost, and is easy for the application in the industrialized production.
Owner:GUIYANG COLLEGE OF TRADITIONAL CHINESE MEDICINE
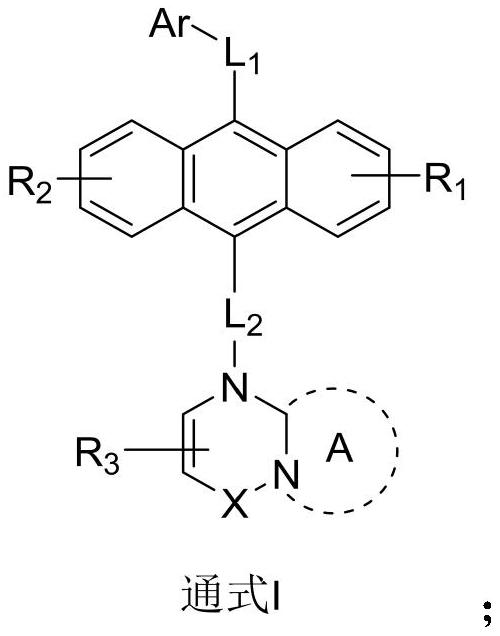
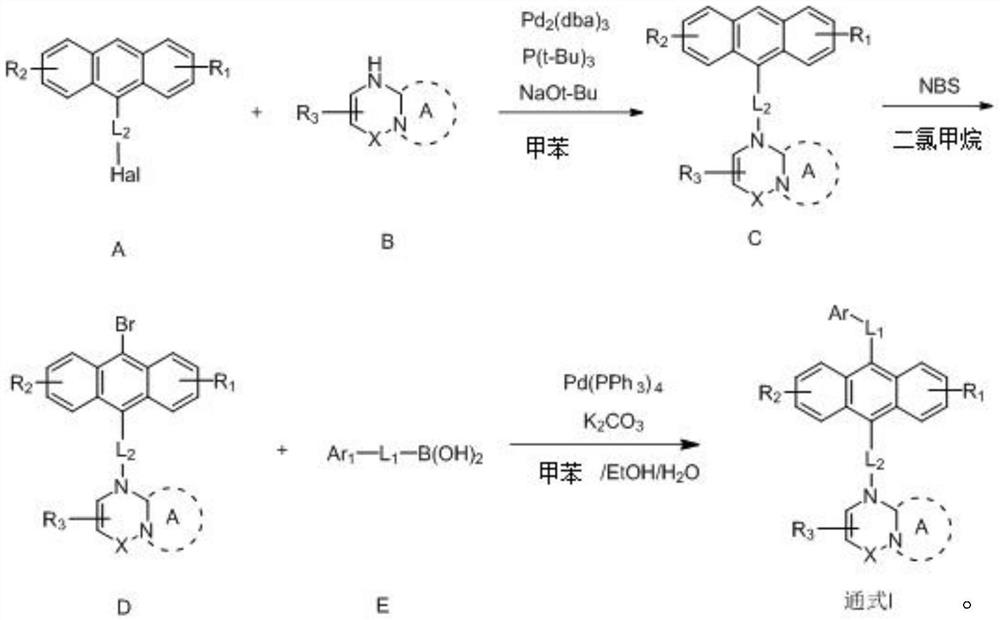
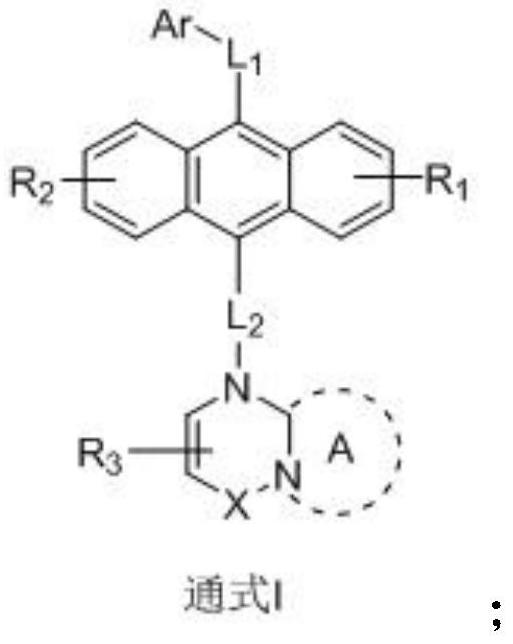
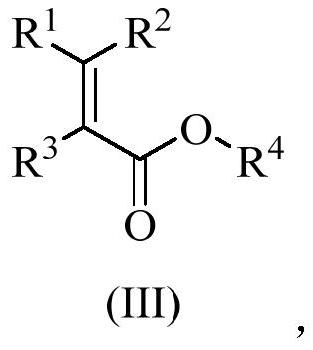
Anthracene nitrogen-containing organic light-emitting compound and preparation method and application thereof
ActiveCN112142672AGood electron transport propertiesImprove luminous performanceOrganic chemistrySolid-state devicesBenzylideneacetoneTert butyl
The invention discloses an anthracene nitrogen-containing organic light-emitting compound and a preparation method and application thereof, the specific structure of the anthracene nitrogen-containingorganic light-emitting compound is as shown in the general formula 1 in the specification, and the preparation method comprises the following steps: taking methylbenzene as a solvent; reacting the raw material A with the raw material B in the presence of sodium tert-butoxide, tris (dibenzylideneacetone) dipalladium and tris(tert-butyl)phosphorus to obtain an intermediate product C, reacting the intermediate product C with NBS by taking dichloromethane as a solvent to obtain an intermediate product D, and reacting the intermediate product D with a mixed solution of methylbenzene, ethanol and water by taking a mixed solution of methylbenzene, ethanol and water as a solvent; and reacting the intermediate product D with a raw material E in the presence of potassium carbonate and tetrakis(triphenylphosphine)palladium to finally obtain the compound shown in the general formula I. The anthracene derivative provided by the invention has relatively high electron transmission performance, and can improve the electron transmission efficiency of a device and can prolong the service life of the device when being applied to an organic electroluminescent device. In addition, the preparation method provided by the invention is simple, easy to operate and suitable for industrial popularization.
Owner:JILIN OPTICAL & ELECTRONICS MATERIALS
Synthesis method of succinic acid derivative or 3-aryl propionic acid
ActiveCN111777477ASelectiveLow toxicityOrganic compound preparationCarboxylic acid esters preparationPropanoic acidButanedioic acid
The invention discloses a synthesis method of a succinic acid derivative or 3-aryl propionic acid. The method comprises the following steps of: adding alkali into a dry reaction tube, adding a solvent, thiophenol and olefin under the atmosphere of CO2, carrying out reaction under the irradiation of visible light, quenching a mixture obtained by reaction after the reaction of raw materials is finished, and then performing separating and purifying to obtain the succinic acid derivative or the 3-aryl propionic acid product; wherein the alkali comprises sodium tert-butoxide, potassium tert-butoxide, lithium tert-butoxide and potassium carbonate; the thiophenol comprises 4-tert-butyl thiophenol and 2, 4, 6-triisopropyl thiophenol; the reaction substrate comprises an acrylate compound or an arylethylene compound. According to the method, the succinic acid derivative and 3-aryl propionic acid can be efficiently and highly selectively synthesized under the induction of visible light and the participation of CO2; according to the scheme, reaction conditions are mild, reaction substrate selectivity is wide, and amplification to a gram-level scale is achieved; raw materials used in the method are cheap and easily available, and the method has good industrial application prospects.
Owner:SICHUAN UNIV
Dimethomorph production method
ActiveCN107935966AHas the disadvantage that the catalytic effect gradually decreasesMake up for the shortcomings of the gradual decrease in catalytic effectOrganic chemistryCatalytic effectBenzophenone
The invention discloses a dimethomorph production method in the technical field of pesticide production. The production method comprises: 1) synthesizing veratrole; 2) synthesizing 3,4-dimethoxy-4-chloro-benzophenone; 3) synthesizing acetylmorpholine; and 4) synthesizing dimethomorph. Sodium tert-butoxide is taken as a basic catalyst for synthesizing dimethomorph from 3,4-dimethoxy-4-chloro-benzophenone and acetylmorpholine, and a sodium hydroxide solution is used to perform alkali washing on a reactant in a reaction vessel. Sodium hydroxide has a catalytic effect on synthesis of dimethomorphfrom 3,4-dimethoxy-4-chloro-benzophenone and acetylmorpholine. The defect that the catalytic effect of sodium tert-butoxide is gradually reduced due to unstable chemical property of sodium tert-butoxide and degradation of sodium tert-butoxide when sodium tert-butoxide comes into water is overcome. The dimethomorph product is improved in conversion rate and purity.
Owner:江苏恒隆作物保护有限公司
Sodium tert-butoxide preparation process
InactiveCN109574800AEasy to manufactureIncrease contact areaPreparation of metal alcoholatesO-XyleneSolvent
The invention discloses a sodium tert-butoxide preparation process. The process includes steps: (1) taking o-xylene as a solvent of a reaction system, adding into a reaction kettle, and adding sodiumblocks into the reaction kettle to melt to obtain a reactant; (2) heating the reaction kettle, adding tert-butyl alcohol, and performing reaction to obtain a crude product of sodium tert-butoxide; (3)subjecting the crude product of sodium tert-butoxide to aftertreatment to obtain a finished product of sodium tert-butoxide. The preparation process has advantages that slicing metal sodium before reaction is avoided, so that a preparation process is simplified, and reaction is facilitated due to increasing of reaction contact area.
Owner:南京大地药业有限公司
Preparation method of meropenem intermediate cyclization compound
ActiveCN103613526ARaw materials are easy to getLow priceOrganic chemistryPotassium tert-butoxideOrganic solvent
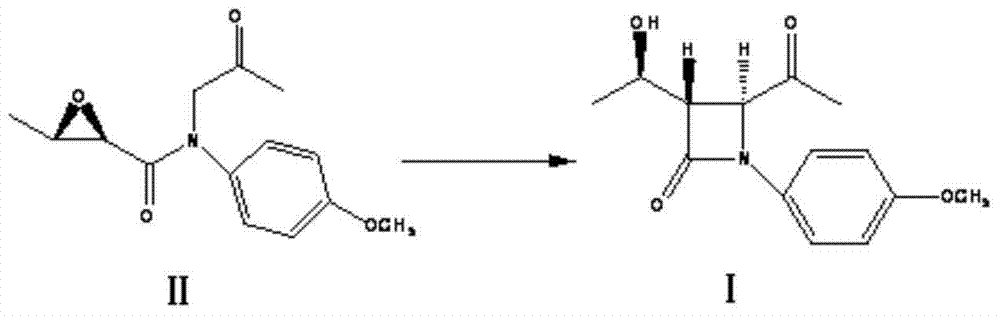
The invention discloses a preparation method for synthesizing an intermediate (3S,4S)-p-methoxyphenyl-3-{(R)-1-hydroxy ethyl}-4-acetyl-2-azetidin-2-one of a meropenem intermediate cyclization compound (3R,4R)-3-{(R)-1-tert-butyl dimethyl silyloxy}-4-acetoxy-2-azetidin-2-one. The method comprises the following steps: dissolving (2R,3R)-N-(p-methoxyphenyl)-N-(2-oxo-propyl)-2,3-epoxy butyrylamide in an organic solvent, and adding lewis acid as a catalyst; cooling; and adding sodium tert-butoxide or potassium tert-butoxide to react so as to obtain the (3S,4S)-p-methoxyphenyl-3-{(R)-1-hydroxy ethyl}-4-acetyl-2-azetidin-2-one. The method disclosed by the invention is low in cost, high in reaction yield, moderate in reaction condition, little in pollution and environment-friendly, and raw materials are easily obtained.
Owner:JIANGSU HANKUO BIOLOGICAL
1-phenyl-2-nitroethanol and preparation method of derivatives thereof
InactiveCN105111086AGood effectHigh conversion rate of synthesis reactionOrganic chemistryOrganic compound preparationNitroethanePtru catalyst
The invention discloses a 1-phenyl-2-nitroethanol and a preparation method of derivatives thereof, and the method employs potassium tert-butoxide or sodium tert-butoxide as a catalyst for catalysis of various benzaldehyde derivatives and nitromethane or nitroethane for preparing 1-phenyl-2-nitroethanol and derivatives thereof. The preparation of the invention has the advantages of good catalyst effect, high synthesis reaction conversion rate, high yield which is 50-90%, low cost of crystallization or extraction method for separating, simple operation, non-pollution due to inorganic base catalysis, and good practicality.
Owner:NANJING UNIV OF INFORMATION SCI & TECH
Sodium tert-butoxide and preparation method thereof
InactiveCN109678663AImprove solubilityQuick responsePreparation of metal alcoholatesTolueneButisol Sodium
The invention discloses sodium tert-butoxide and a preparation method thereof. The preparation method comprises the following steps: (1) taking methylbenzene or heptanes as a reaction medium, adding sodium amide and tertiary butanol, mixing and stirring to dissolve to obtain a mixed solution; (2) heating the mixed solution to 70-110 DEG C for reaction to obtain a crude product of sodium tert-butoxide till the reaction is completed; (3) rectifying the crude product of the sodium tert-butoxide to obtain a finished product of the sodium tert-butoxide. The preparation method disclosed by the invention has relaxed reaction conditions, is small in danger in the production process, simple in aftertreatment process of products and short in drying time, and the product yield is up to 99% or above.
Owner:南京大地药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method for preparing compound 2-(imidazo [1, 2-a] pyridine-3-yl) acetonitrile Method for preparing compound 2-(imidazo [1, 2-a] pyridine-3-yl) acetonitrile](https://images-eureka.patsnap.com/patent_img/1bcb5027-c3fb-471e-86b4-f3fbc755948e/DSA00000410367500011.png)
![Method for preparing compound 2-(imidazo [1, 2-a] pyridine-3-yl) acetonitrile Method for preparing compound 2-(imidazo [1, 2-a] pyridine-3-yl) acetonitrile](https://images-eureka.patsnap.com/patent_img/1bcb5027-c3fb-471e-86b4-f3fbc755948e/FSA00000410367600011.png)
![Method for preparing compound 2-(imidazo [1, 2-a] pyridine-3-yl) acetonitrile Method for preparing compound 2-(imidazo [1, 2-a] pyridine-3-yl) acetonitrile](https://images-eureka.patsnap.com/patent_img/1bcb5027-c3fb-471e-86b4-f3fbc755948e/BSA00000410367700011.png)