Preparation method of meropenem intermediate cyclization compound
A technology for temperature control and azetidinone, which is applied in the field of preparation of meropenem intermediates, can solve the problems of pollution and many metal-containing acidic wastewater, and achieves the effects of less wastewater, low price and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
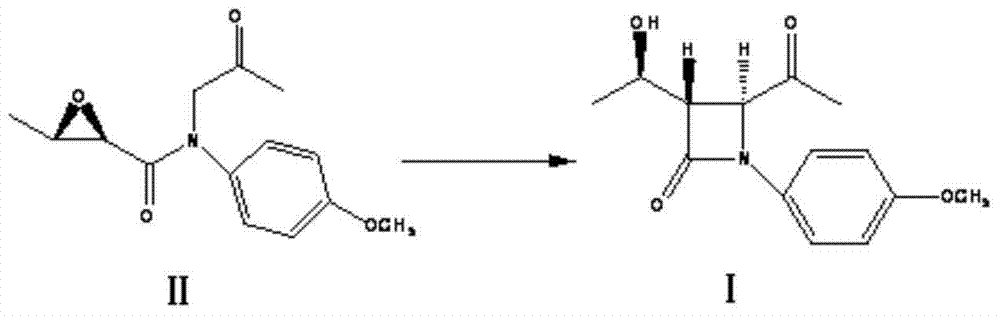
[0026] Dissolve 0.36mol of (2R,3R)-N-(p-methoxyphenyl)-N-(2-oxo-propyl)-2,3-epoxybutyramide in tetrahydrofuran, add lithium chloride 0.05mol, lower the temperature to -10~0°C, add 0.72mol of sodium tert-butoxide to react, control the reaction temperature at 0°C, after the reaction is completed, concentrate, add 300ml of dichloromethane to the residue, add 400mL of 1N HCl solution and stir Wash for 10 min, separate the layers, wash the organic layer with saturated sodium bicarbonate until neutral, wash with saturated brine, and concentrate to remove the solvent to obtain (3S,4S)-1-p-methoxyphenyl-3-[(R)- 92 g of 1-hydroxyethyl]-4-acetyl-2-azetidinone. Yield: 82.4%, HPLC purity ≥ 85%.
[0027] The structure confirmation data of the product: 1 H-NMR (500MHz, CDCl 3 δ): 1.42(d,J=6.37Hz,3H),1.64(brs,1H),2.28(S,3H),3.2(dd,J=2.64and5.26Hz,1H),3.81(S,3H), 4.38(M,1H),4.58(d,J=2.64Hz,1H),6.90(d,J=6.87Hz,2H),7.25(d,J=6.8Hz,2H).
Embodiment 2
[0029] Dissolve 0.36mol of (2R,3R)-N-(p-methoxyphenyl)-N-(2-oxo-propyl)-2,3-epoxybutanamide in methyl tert-butyl ether , add 0.05mol of zinc chloride, lower the temperature to 0-10°C, add 0.76mol of potassium tert-butoxide, control the temperature at 20°C, after the reaction is completed, concentrate, add 300ml of dichloromethane to the residue, and add 500mL of 1N HCl solution Stir and wash for 10 min, separate layers, wash the organic layer with saturated sodium bicarbonate until neutral, wash with saturated brine, and concentrate to remove the solvent to obtain (3S,4S)-1-p-methoxyphenyl-3-[(R) - 91 g of 1-hydroxyethyl]-4-acetyl-2-azetidinone. Yield: 81.5%, HPLC purity ≥ 85%.
[0030] The structure confirmation data of the product: 1 H-NMR (500MHz, CDCl 3 δ): 1.42(d,J=6.37Hz,3H),1.64(brs,1H),2.28(S,3H),3.2(dd,J=2.64and5.26Hz,1H),3.81(S,3H), 4.38(M,1H),4.58(d,J=2.64Hz,1H),6.90(d,J=6.87Hz,2H),7.25(d,J=6.8Hz,2H).
Embodiment 3
[0032] Dissolve 0.36mol of (2R,3R)-N-(p-methoxyphenyl)-N-(2-oxo-propyl)-2,3-epoxybutanamide in toluene, add zinc chloride 0.05mol, lower the temperature to 0-10°C, add 1.08mol of sodium tert-butoxide, control the temperature at 50°C, after the reaction is completed, concentrate, add 300ml of dichloromethane, add 600mL of 1N HCl solution, stir and wash for 10min, separate layers , the organic layer was washed with saturated sodium bicarbonate until neutral, washed with saturated brine, and concentrated to remove the solvent to obtain (3S,4S)-1-p-methoxyphenyl-3-[(R)-1-hydroxyethyl ]-4-acetyl-2-azetidinone 83 g. Yield: 74.3%, HPLC purity ≥ 85%.
[0033] The structure confirmation data of the product: 1 H-NMR (500MHz, CDCl 3 δ): 1.42(d,J=6.37Hz,3H),1.64(brs,1H),2.28(S,3H),3.2(dd,J=2.64and5.26Hz,1H),3.81(S,3H), 4.38(M,1H),4.58(d,J=2.64Hz,1H),6.90(d,J=6.87Hz,2H),7.25(d,J=6.8Hz,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com