1-phenyl-2-nitroethanol and preparation method of derivatives thereof
A technology of nitroethanol and derivatives, which is applied in the field of preparation of 1-phenyl-2-nitroethanol and its derivatives, can solve the problems of unsuitable purification of products, unsatisfactory catalytic effect, low yield, etc. Low cost, good practicability and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of 1-(2,4-dichloro)phenyl-2-nitroethanol
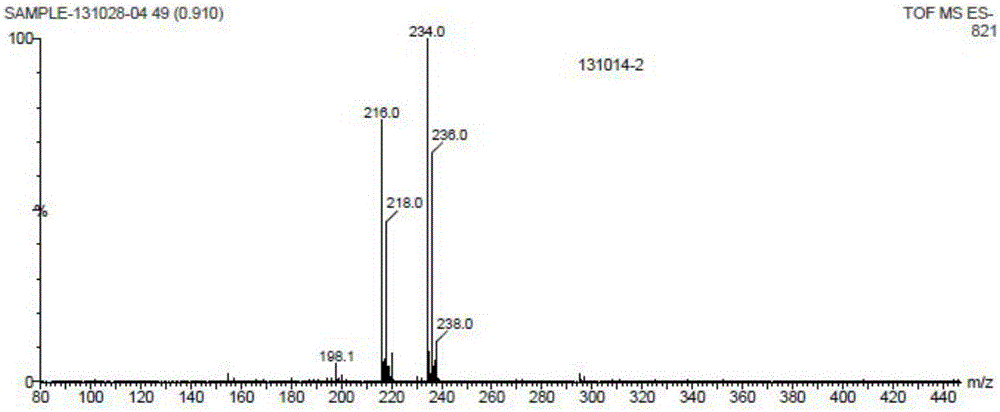
[0037] Add 15mL t-BuONa / t-BuOH solution (containing t-BuONa2mmol) and 15mLTHF into a 100mL flask, add 10.8mL (0.2mol) nitromethane under stirring, after stirring for a while, add 3.5g (20mmol) 2,4 -Dichlorobenzaldehyde, stirred at room temperature for 5h. After TLC (thin layer chromatography) monitored the completion of the reaction, 30 mL of saturated ammonium chloride solution and 40 mL of ethyl acetate were added and stirred for 10 minutes. After liquid separation, extract the aqueous phase with 30mL*2 ethyl acetate, combine the organic phases, wash twice with 30mL saturated sodium sulfate solution, dry over anhydrous magnesium sulfate, filter, and evaporate the solvent to obtain a nearly colorless oily substance, which is kept at room temperature After 4 hours, the product crystallized and was vacuum-dried at room temperature to obtain 4.2 g of a yellowish powdery solid with a yield of 90%. The m.p. (melting po...
Embodiment 2
[0039] Preparation of 1-phenyl-2-nitroethanol
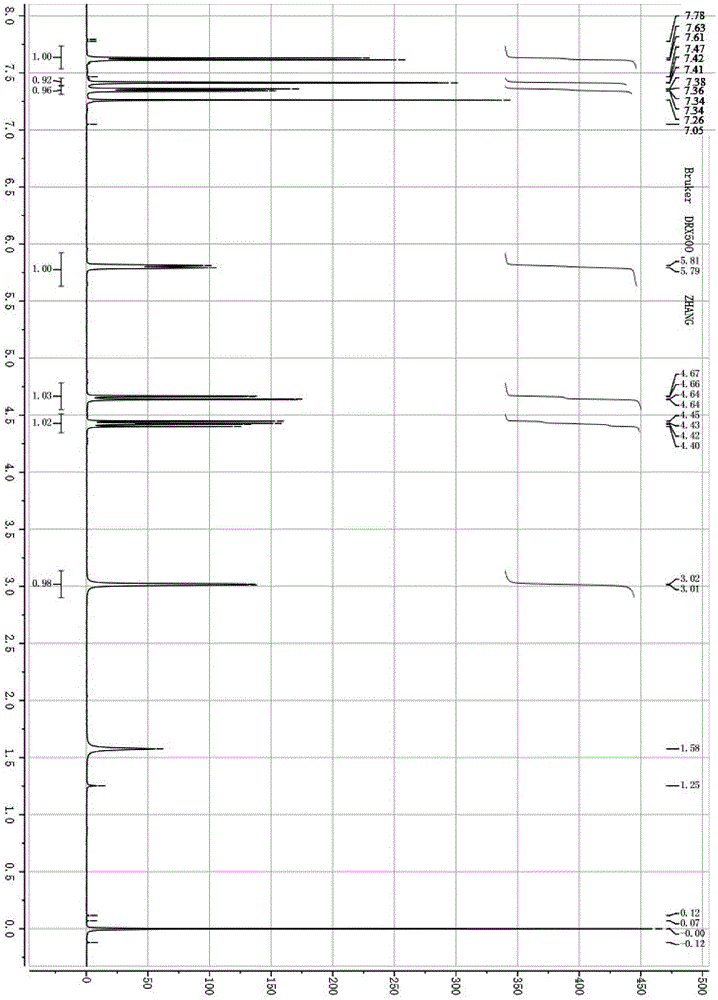
[0040] 2.1g (20mmol) of benzaldehyde was used instead of 3.5g (20mmol) of 2,4-dichlorobenzaldehyde to react with nitromethane. The synthesis method was the same as in Example 1. The product was an oily substance with a yield of 70%. 1 HNMR (500MHz, CDCl 3 ) analysis result is (see Figure 4 ): δ7.41–7.35(m,5H,Ar-H),5.45(dd, J =10,3Hz,1H,CH-O),4.60(dd, J =13.5Hz,10Hz,1H,-CH 2 -),4.51(dd, J =13.5Hz,3Hz,1H,-CH 2 -), 2.73 (bs, 1H, -OH). The obtained oil was proved to be 1-phenyl-2-nitroethanol.
Embodiment 3
[0042] Preparation of 1-(3-nitro)phenyl-2-nitroethanol
[0043] Add 0.03 g of t-BuOK (2.7 mmol), 5 mL of t-BuOH / THF, and 1.5 mL (28 mmol) of nitromethane into a 100 mL flask, and stir well. Dissolve 0.42g (2.8mmol) of m-nitrobenzaldehyde in 1.5mL of THF, add dropwise into a one-necked bottle, and react under normal temperature and pressure. TLC tracking detection, after the reaction is complete, add 20mL distilled water and 30mL EtOAc (ethyl acetate) to the system, stir and separate the liquid, the aqueous phase is extracted twice with 20mL EtOAc, after combining the organic phase, the organic phase is washed twice with 30mL saturated NaCl, no Water MgSO 4After drying, the solvent was evaporated to give 0.95 g of a pale yellow oil. Recrystallization and purification with PE gave 0.51 g of light yellow solid. Yield 86%. Pale yellow solid m.p. (melting point) is 75-76 oC . 1 HNMR (500MHz, CDCl 3 ) analysis result is (see Figure 5 ): δ8.32(s,1H,Ar-H),8.23(d,1H, J =14Hz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com