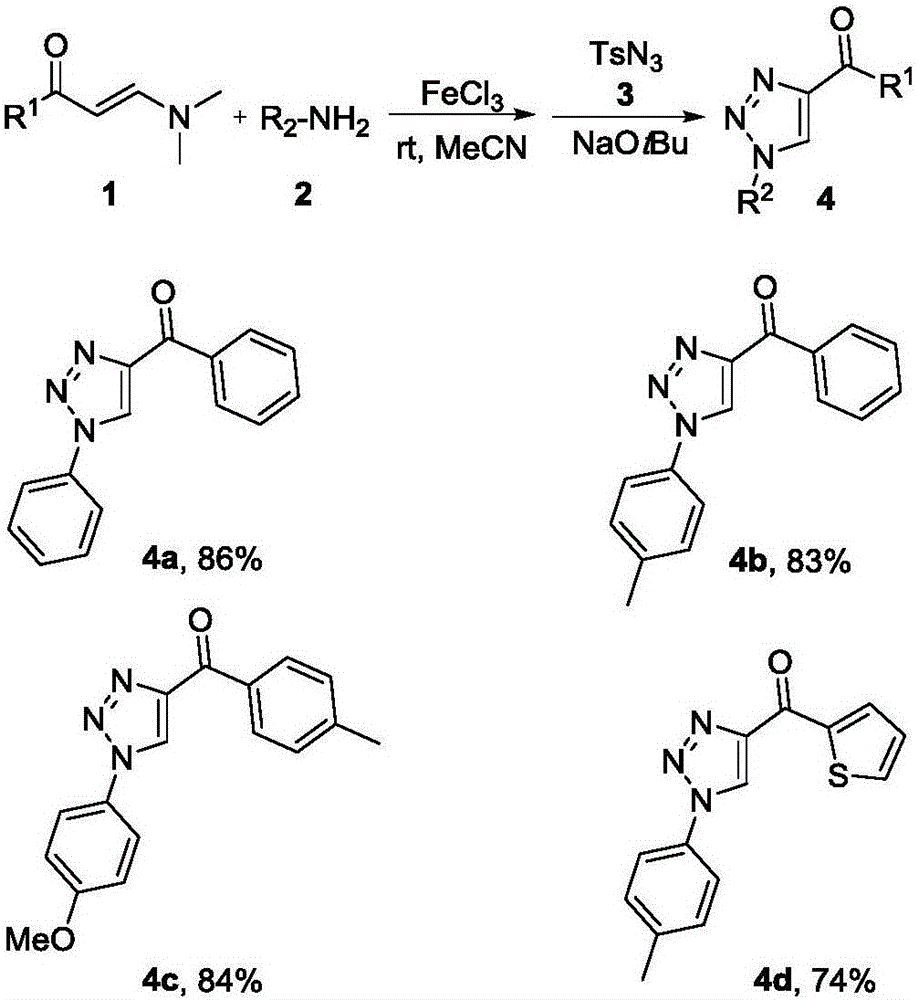
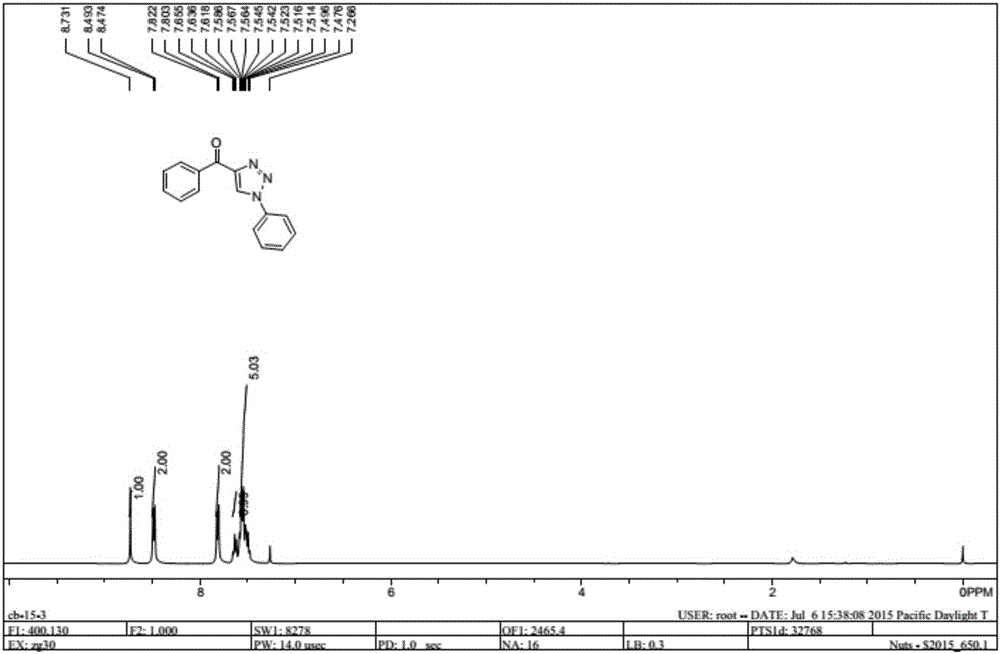
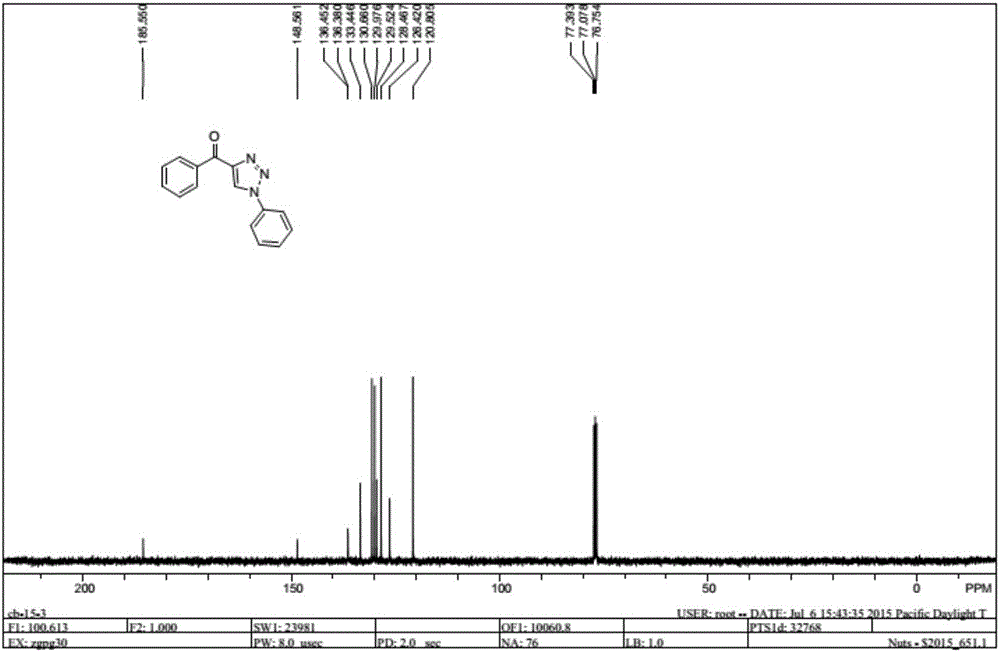
Method for synthesizing 1, 4-bis-substituted-1, 2, 3-triazole by cycloaddition reaction of base catalysis enaminone and sulfonyl azide
A technology of base-catalyzed enaminones and sulfonyl azide rings, which is applied in the direction of organic chemistry and the like, and achieves the effects of cheap and easy-to-obtain raw materials, wide application prospects, and environmental friendliness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] The present invention is achieved by N,N-dimethyl substituted amino enaminone 1 (0.3mmol), primary amine 2 (0.4mmol) and FeCl 3 (0.15 mmol) was placed in a dry 25 mL round bottom flask, then 2 mL of acetonitrile was added, and stirred at room temperature for 2 hours. Sulfonyl azide 3 (0.4 mmol) and sodium tert-butoxide (0.45 mmol) were then added and stirring was continued at room temperature for 2 hours. After the reaction was completed, 5 mL of water was added to the flask, and the resulting mixture was extracted with ethyl acetate (3×8 mL), and the organic phases were combined and dried over anhydrous magnesium sulfate. After filtration, the solvent in the filtrate was removed under reduced pressure, and the residue was purified by silica gel column chromatography and washed with ethyl acetate:petroleum ether (6:1) mixture to obtain the target product 4. The structure and purity of all products were confirmed by nuclear magnetic resonance, high-resolution mass spect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com