Preparation method of 2-trifluoromethyl indole derivatives
A technology of trifluoromethyl indole derivatives and groups, which is applied in the field of 2-position trifluoromethyl indole derivatives and their synthesis, and can solve problems such as poor selectivity, few product types, and low atom economy. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
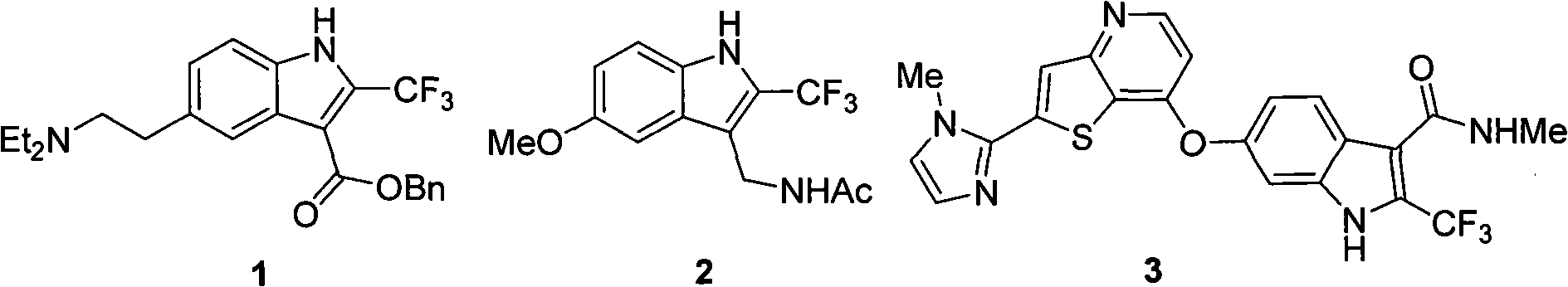
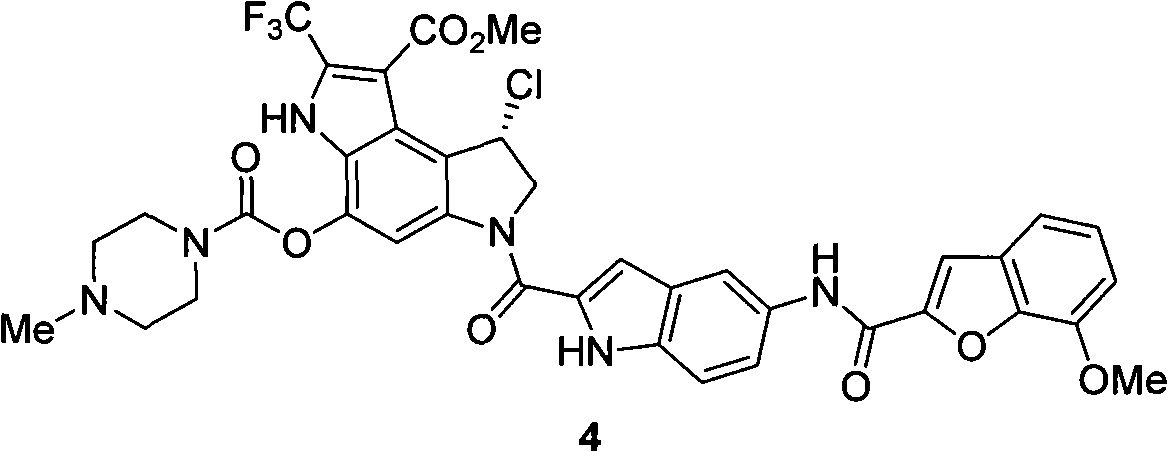
[0030] In order to further specify the synthesis method of the trifluoromethyl indole derivatives with two positions, the implementation methods are specifically stated here.
[0031] Preparation of two-position trifluoromethyl indole derivative B:
[0032] Compound A (0.2mmol), primary amine (0.24mmol), [Pd] (10mol%), N-P ligand (20mol%), sodium tert-butoxide (4equiv) were placed in a 25mL round tube with polyvinyl fluoride seal In the bottom flask, inject toluene (3mL) then, seal the flask, react at reflux temperature for 3-32 hours, extract with ethyl acetate after the completion of the reaction, collect the organic layer, dry, concentrate, and purify to obtain the compound with excellent yield.
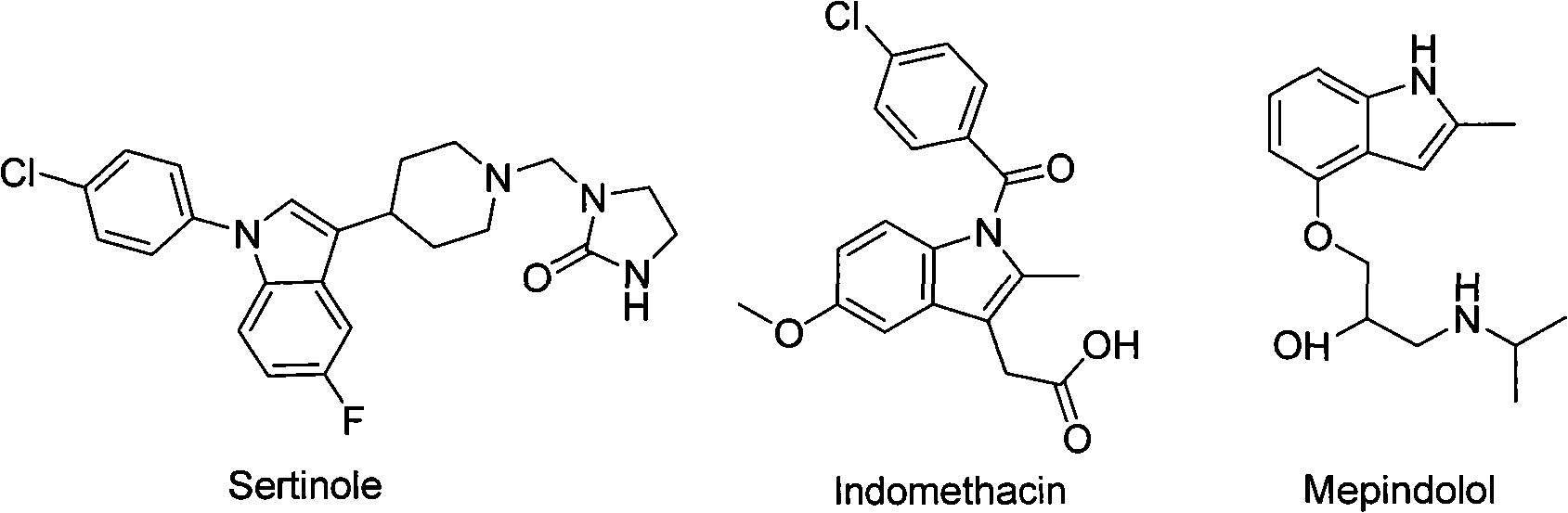
[0033] In order to further elaborate the synthesis method of the trifluoromethyl sertindole derivative with the two-position, its implementation mode is specifically stated here.
[0034] Preparation of the two-position trifluoromethyl sertindole derivative C:
[0035] 2-CF 3 I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com