Synthetic method of cholesterol
A synthetic method, cholesterol technology, applied in the direction of steroids, organic chemistry, etc., can solve the problems of large consumption of raw and auxiliary materials, long steps, uneconomical, etc., and achieve the effect of less raw and auxiliary materials, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
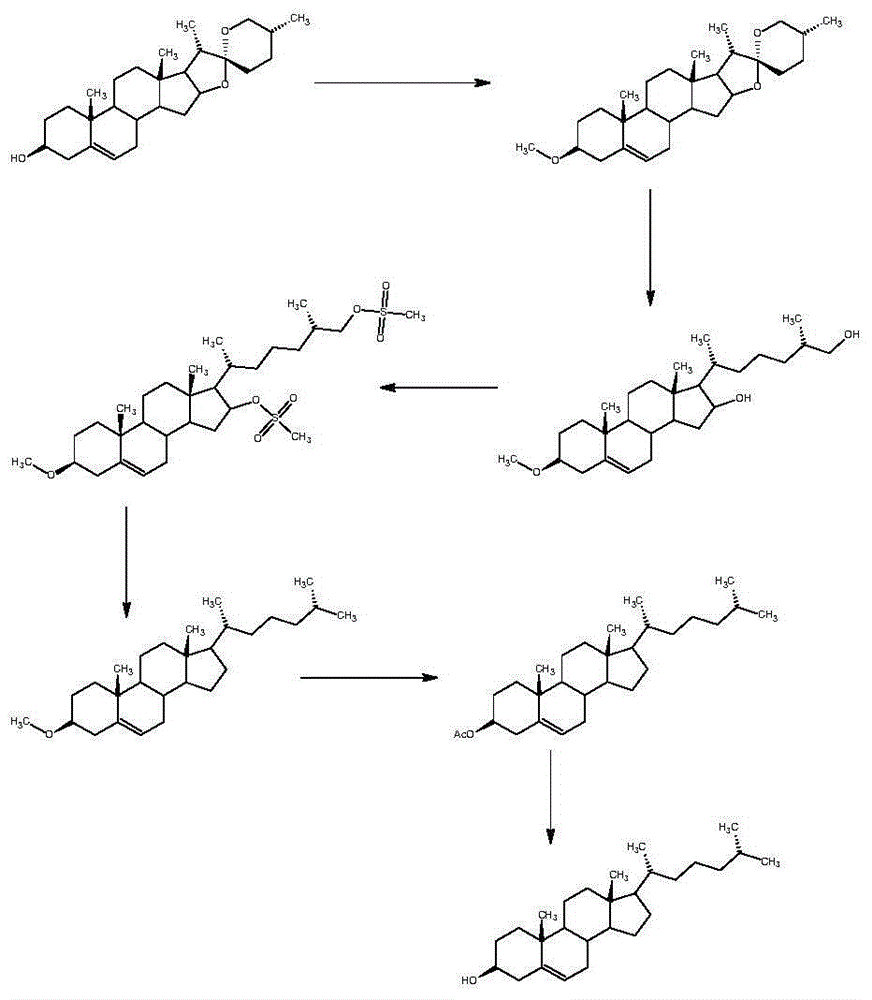
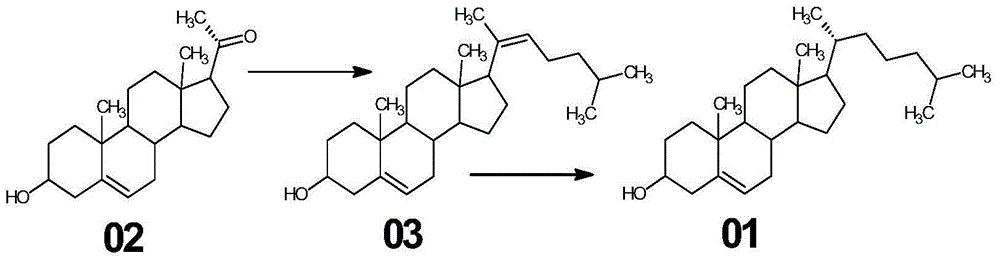
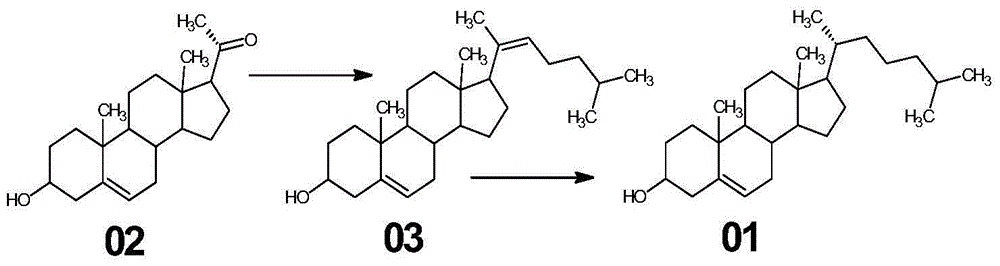
[0032] The present embodiment is the synthetic method of cholesterol, specifically comprises the following steps:
[0033] (1) Synthesis of compound 03
[0034] (a) In a 2000ml glass reaction bottle, add 1200ml of toluene, stir, add 63g of triphenylphosphine, heat to reflux, steam and divide water until the toluene is clear, stop heating, cool down to below 40°C under nitrogen protection, add 1-chloro - 29 g of 4-methylpentane, heated under reflux for 2 hours to obtain 4-methylpentyltriphenylphosphine chloride solution;
[0035] (b) The 4-methylpentyltriphenylphosphine chloride solution is cooled under nitrogen protection to below 20°C, and 32 g of potassium tert-butoxide is added in stages to obtain the witting reagent, and after stirring for 30 minutes, the witting reagent is added Pregnenolone 50g, after adding, heat and reflux reaction for 4 hours, after the plate reaction is complete;
[0036] (c) ice-water bath is cooled to below 10 ℃, slowly changes in the acid soluti...
Embodiment 2
[0040] The difference between this embodiment and Example 1 is that in this embodiment, triphenylphosphine, 1-chloro-4-methylpentane, potassium tert-butoxide, 30% industrial concentrated The amount of hydrochloric acid was adjusted to 94g, 44g, 48g and 58g respectively to finally obtain compound 0354.66g with a molar yield of 89.96%.
Embodiment 3
[0042] The difference between this example and Example 1 is that in this example, the amount of triphenylphosphine, 1-chloro-4-methylpentane, and potassium tert-butoxide in step (1) is adjusted to 125g respectively , 58g, 48g and 64g, 30% industrial concentrated hydrochloric acid was replaced by 32g concentrated sulfuric acid, finally compound 0354.88g was obtained, and the molar yield was 90.32%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com