Preparation method of multi-substituted thiochromanone derivative
A technology for thiochromanone and its derivatives, which is applied in the field of preparation of multi-substituted thiochromanone derivatives, can solve the problems of harsh conditions, cumbersome synthesis steps, unfriendly environment, etc., and achieve easy purification, simple synthesis method, and synthesis The scientific and reasonable effect of the method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1) Preparation of Thiochromanone Derivative 3a
[0030]
[0031] Add β-carbonyldithiocarboxylate methyl ester 2a (0.5mmol, 105mg), CuI (0.5mmol, 95mg), o-phenanthroline (0.5mmol, 90.1mg) and t-BuONa (2.0mmol ,192mg). Add toluene (1.5mL), pre-stirrer at 80°C under nitrogen atmosphere for 5 minutes, slowly drop o-bromoacetophenone 1a (1.0mmol, 190mg) into the system, finish the drop within 5 minutes, and continue the reaction for 4 hours. After the reaction is complete, cool to room temperature, add dilute hydrochloric acid (1M) to adjust to weak acidity, then extract 3 times with ethyl acetate, wash the organic phase with saturated NaCl and wash with anhydrous MgSO 4 Dry for 30 minutes, remove the solvent with a rotary evaporator, and separate the residue by column chromatography (200-300 mesh silica gel) (petroleum ether / ethyl acetate=5 / 1) to obtain a yellow solid thiochromanone derivative 3a. The rate is 82%.
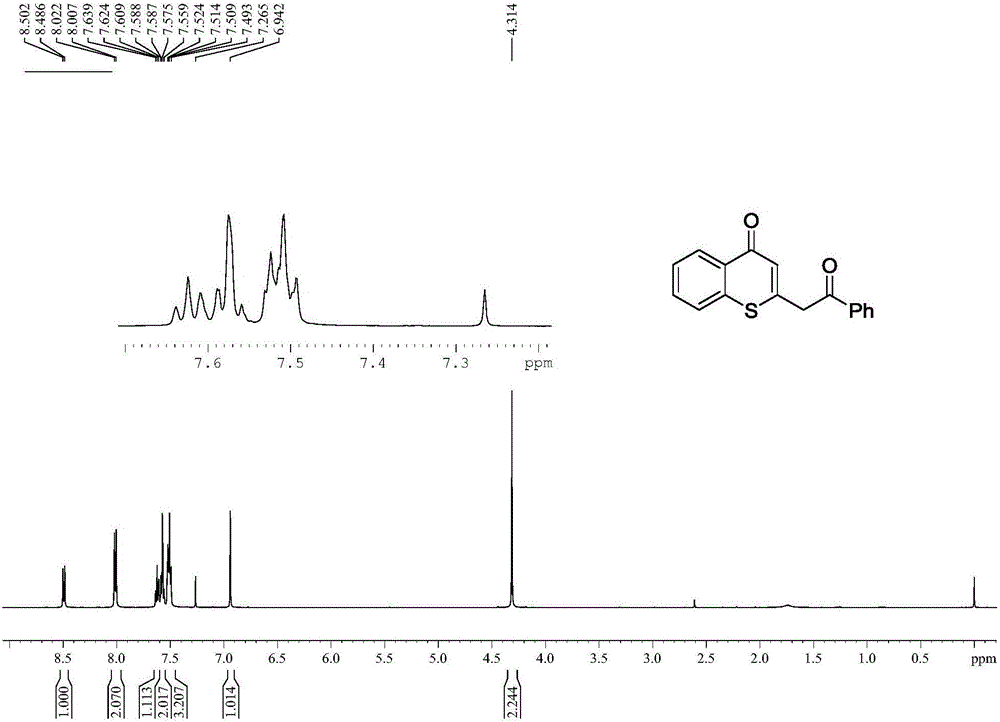
[0032] Spectral analysis data 3a:
[0033] 1 H NMR...
Embodiment 2
[0035] Replace 2a in Example 1 with 2b, and other conditions are the same as Example 1. The experimental results are shown in Table 1.
[0036]
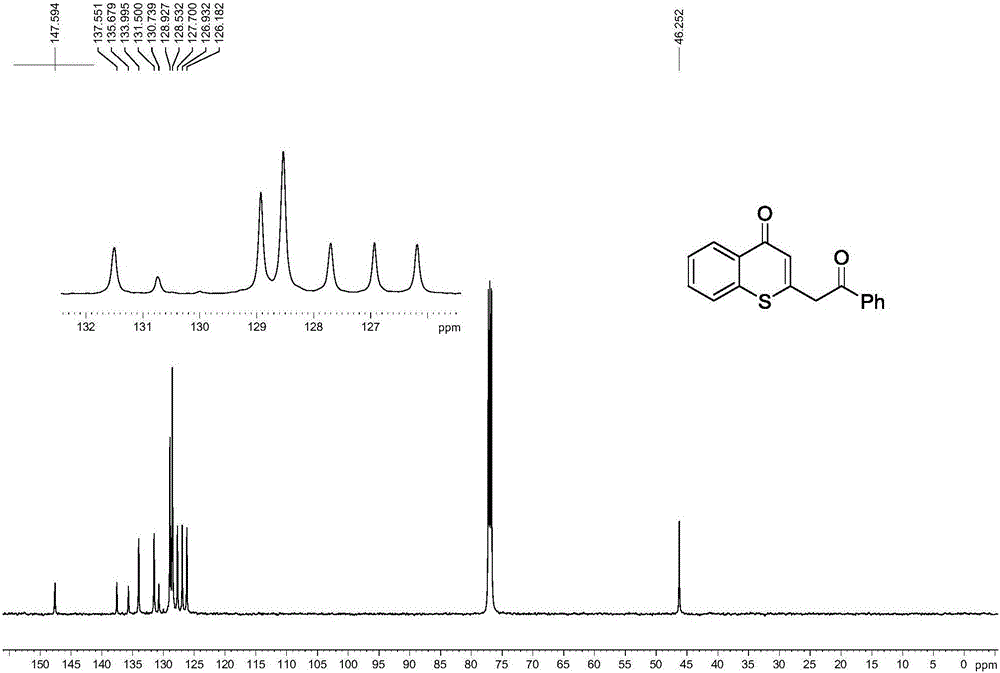
[0037] Spectrum analysis data 3b:
[0038] 1 H NMR (CDCl 3 ,500MHz)δ:4.32(s,2H),6.92(s,1H),7.34-7.37(m,1H),7.44-7.46(m,2H),7.52-7.53(m,1H),7.57-7.60( m,2H),8.45(d,J=8.0Hz,1H); 13 C NMR (CDCl 3 ,125MHz)δ:50.0,126.2,127.0,127.2,127.7,128.5,129.5,130.7,131.5,132.6,137.5,137.8,146.8,180.3,196.5; HRMS(ESI-TOF):calcd for C 17 h 12 o 2 SCl[M+H] + :315.0247,found:315.0250.
Embodiment 3
[0040] Replace 2a in Example 1 with 2c, and other conditions are the same as Example 1. The experimental results are shown in Table 1.
[0041]
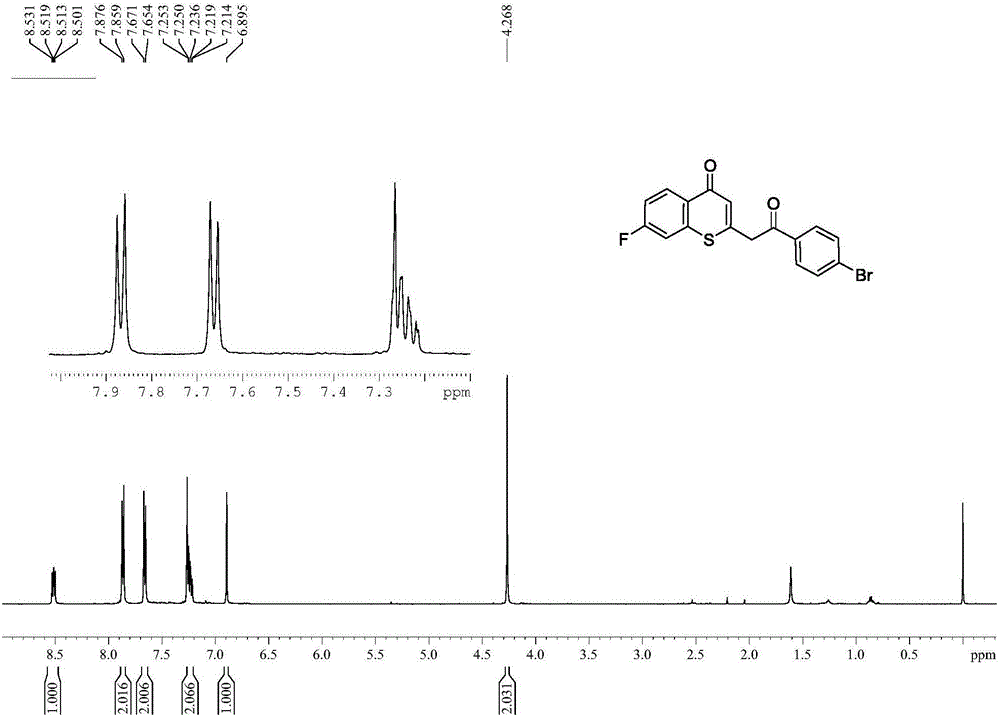
[0042] Spectrum analysis data 3c:
[0043] 1 H NMR (CDCl 3,500MHz)δ:4.27(s,2H),6.89(s,1H),7.21-7.25(m,2H),7.66(d,J=8.4Hz,2H),7.87(d,J=8.45Hz,2H ),8.52(dd, 1 J=8.85Hz, 2 J=5.9Hz,1H); 13 C NMR (CDCl 3 ,125MHz)δ:45.6,45.7,112.1(d, 2 J C-F =24.48Hz), 116.5(d, 2 J C-F =22.29Hz), 127.1, 127.4, 129.6, 129.9, 131.7 (d, 3 J C-F =8.98Hz), 132.4, 134.3, 139.5, 146.8, 164.1 (d, 1 J C-F =256.39Hz), 179.3, 192.5; HRMS (ESI-TOF): calcd for C 17 h 11 o 2 FSBr[M+H] + :376.9647,found:376.9649.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com