Silca bridged cycloalkene compounds and preparation method and use thereof
A technology for silacyclopentene and compounds, which is applied in the field of important organic synthesis intermediates, can solve the problems of no literature reports and no synthesis methods in the research of silacyclopentene, and achieve simple, feasible and applicable scope of experimental equipment and operation The effect of wide and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
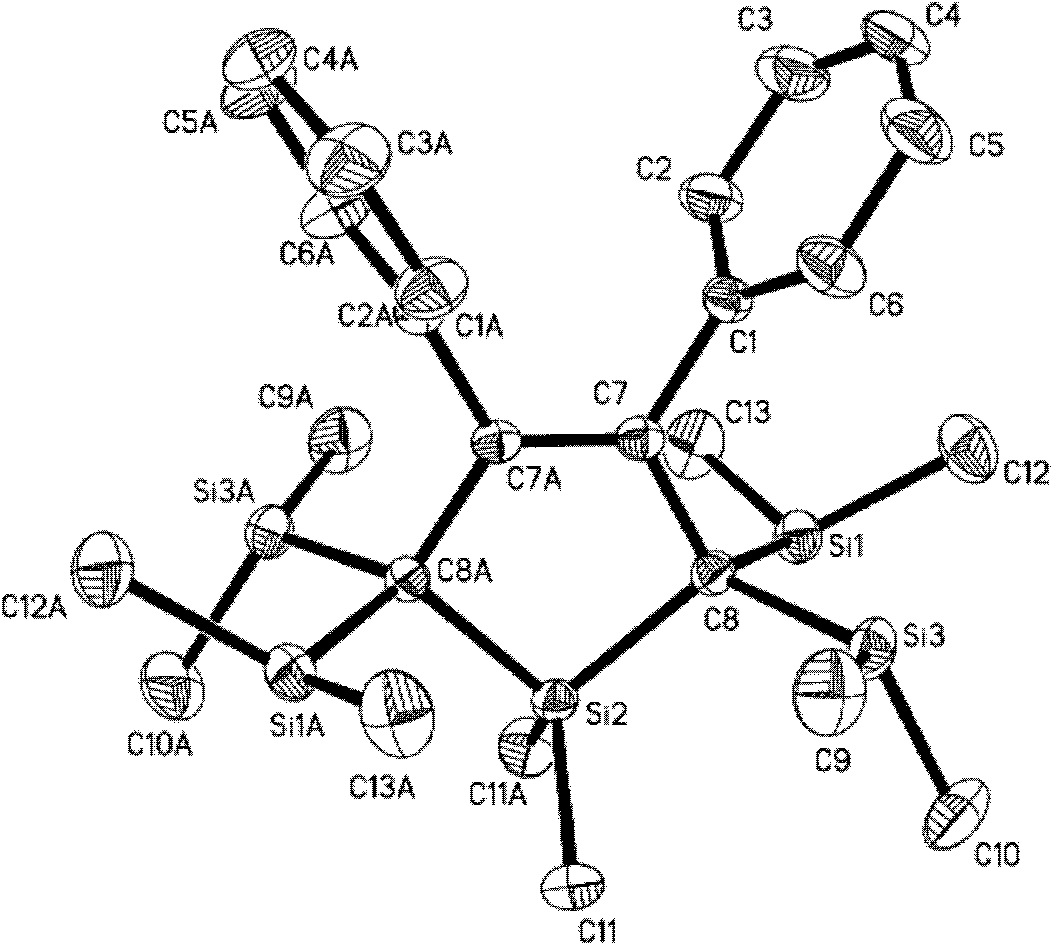
[0052] Example 1: Preparation of 1,1 dialkyl(aryl)yl-3,4-diphenyl-2,5-dilithium silole.
[0053] Taking 1,1 dimethyl-3,4-diphenyl-2,5-dilithium silole as an example, prepared according to the method disclosed in the literature (J AmChem Soc, 1994, 116:11715211722.), under the protection of nitrogen Next, in a 50ml reaction flask, lithium metal (0.14g, 0.02mol) was reacted with naphthalene (2.56g, 0.02mol) dissolved in 12mlTHF at room temperature for 3 hours. Add dropwise dimethyldiphenylethynylsilane (1.30 g, 0.005 mol) dissolved in 5 ml THF to the above solution, and react for 1 hour to generate 1,1 dimethyl-3,4-diphenyl-2,5 - Dilithium silole. It was directly used in the next reaction without separation.
Embodiment 2
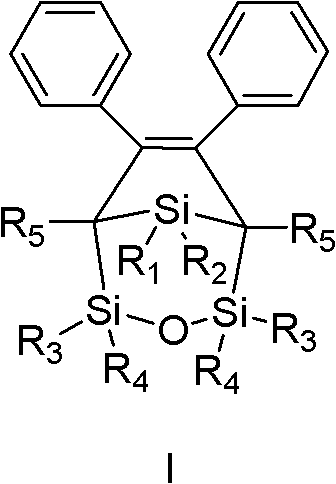
[0054] Example 2: Preparation of 1,1-dimethyl-3,4-diphenyl-2,2,5,5-tetrakis(dimethylsilyl)yl-3-silacyclopentene.
[0055]
[0056] R 1
R 2
R 3
R 4
R 5
Methyl
Methyl
Methyl
Methyl
---
[0057] At room temperature, under the protection of nitrogen, metal lithium (0.28g, 0.04mol) reacted with naphthalene (5.12g, 0.04mol) dissolved in 24mlTHF for 3 hours at room temperature, and then added 2.6g of dimethyl dissolved in 10mlTHF Diphenylethynylsilane (0.01mol), reacted for 1 hour to obtain a reaction solution containing 0.01mol 1,1-dimethyl-3,4-diphenyl-2,5-dilithium silole, and then added 4.0ml Dimethylchlorosilane (0.04mol), reacted for 10 hours, extracted the aqueous phase with 25ml ether three times after the reaction was completed, combined the organic phases, washed with saturated sodium chloride solution, and washed with anhydrous MgSO 4 Dry for half an hour, filter under normal pressure, concentrate the f...
Embodiment 3
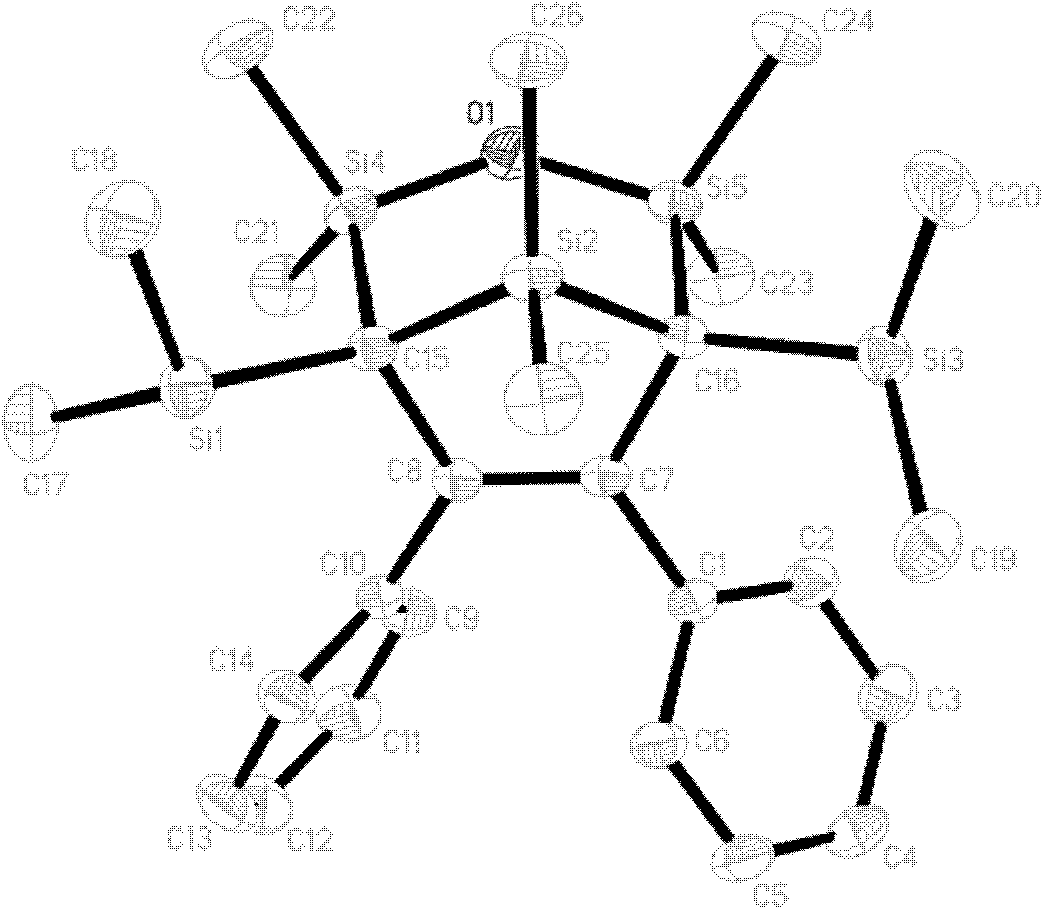
[0061] Example 3: Preparation of 1,1,3,4-tetraphenyl-2,2,5,5-tetrakis(methylphenylsilyl)yl-3-silacyclopentene.
[0062]
[0063] R 1
R 2
R 3
R 4
R 5
Phenyl
Phenyl
Methyl
Phenyl
---
[0064] At 25°C, under the protection of nitrogen, metal lithium (0.28g, 0.04mol) reacted with naphthalene (5.12g, 0.04mol) dissolved in 24mlTHF for 3 hours at room temperature, and then added 3.92g diphenyl diphenylene dissolved in 10mlTHF Diphenylethynylsilane (0.01mol), reacted for 1 hour to prepare a reaction solution containing 0.01mol 1,1,3,4-tetraphenyl 2,5-dilithium silole, and then added 5.0ml methylbenzene Chlorochlorosilane (0.05mol), reacted for 10 hours, extracted the aqueous phase three times with 25ml ether after the reaction was completed, combined the organic phases, washed with saturated sodium chloride solution, and dried for half an hour with anhydrous MgSO4 after liquid separation, filtered under normal press...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com