Preparation method of poly-substituted diphenylethylene indole derivative
A technology of stilbene derivatives and stilbene, which is applied in the field of preparation of stilbene stilbene derivatives, can solve problems such as long reaction time, and achieve short reaction time, high yield, and high environmental friendliness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
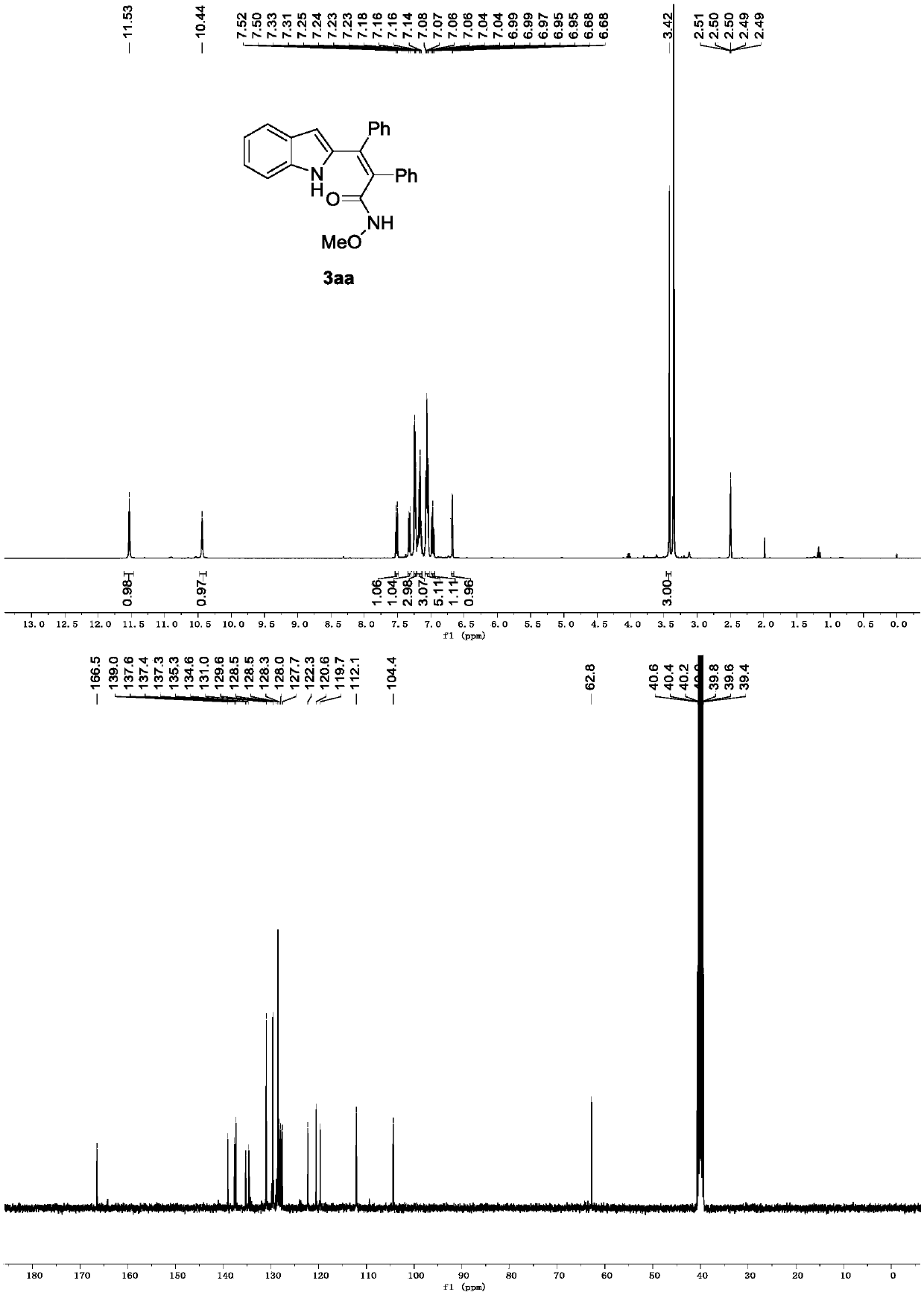
[0021] Preparation of Distyrylindole Derivative 3aa
[0022]
[0023] Add N-methoxyindolecarboxamide 1a (0.1mmol, 19.0mg), 2a (0.1mmol, 17.8mg), dichlorobis(4-methylisopropylphenyl) into a 15ml thick-walled pressure-resistant tube Ruthenium(Ⅱ) (0.005mmol, 3.1mg) and sodium acetate (0.2mmol, 27.2mg) were added to water:dichloromethane (1.0mL, 9:1), stirred at room temperature, and reacted for 3 hours. After the reaction is complete, use a rotary evaporator to remove the solvent to obtain a crude product, which is separated by column chromatography (200-300 mesh silica gel) (petroleum ether / ethyl acetate=4 / 1), and uses a rotary evaporator to remove the solvent to obtain the target The yield of the unsubstituted distyrylindole derivative 3aa was 99%.
[0024] Spectrum analysis data 3aa:
[0025] 1 H NMR (400MHz, DMSO-d 6 )δ11.53(s,1H),10.44(s,1H),7.51(d,J=7.8Hz,1H),7.32(d,J=8.1Hz,1H),7.24(dd,J=5.0,1.7 Hz,3H),7.20–7.13(m,3H),7.09–7.02(m,5H),7.00–6.94(m,1H),6.68(d,J=1.5Hz,1...
Embodiment 2
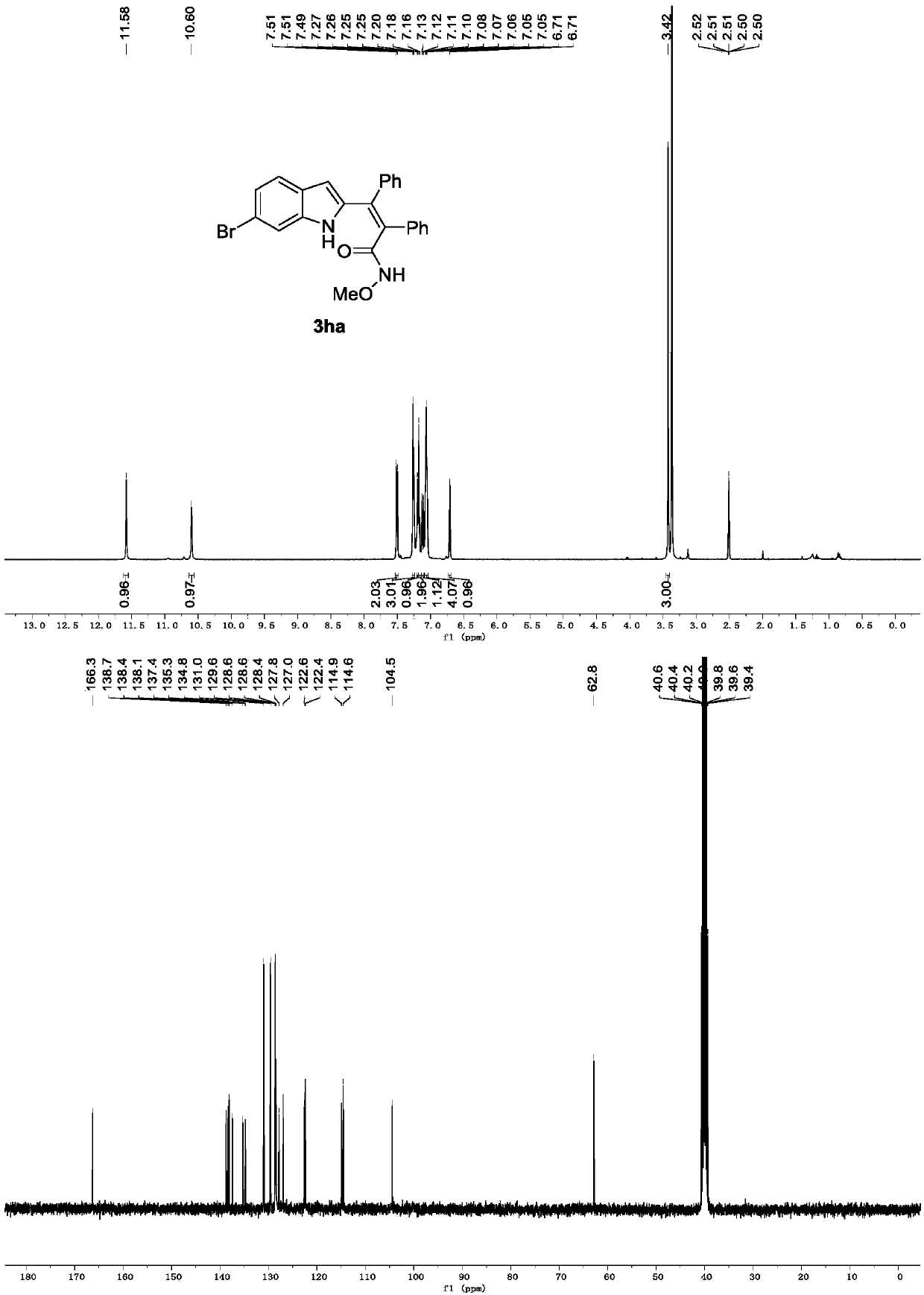
[0027] Replace 1a in Example 1 with 1b, and other conditions are the same as Example 1. The experimental results are shown in Table 1.
[0028]
[0029] Spectrum analysis data 3ba:
[0030] 1 H NMR (400MHz, DMSO-d 6 )δ11.57(s,1H),10.68(s,1H),7.73(d,J=1.7Hz,1H),7.29(d,J=8.6Hz,1H),7.24(dd,J=5.0,1.6 Hz,3H),7.18(d,J=1.9Hz,2H),7.16(d,J=3.0Hz,2H),7.09–7.02(m,4H),6.68(d,J=1.6Hz,1H), 3.42(s,3H). 13 C NMR (101MHz, DMSO-d 6 ( 24 h 19 BrN 2 NaO 2 + [M+Na] + 469.0528,found 469.0528.
Embodiment 3
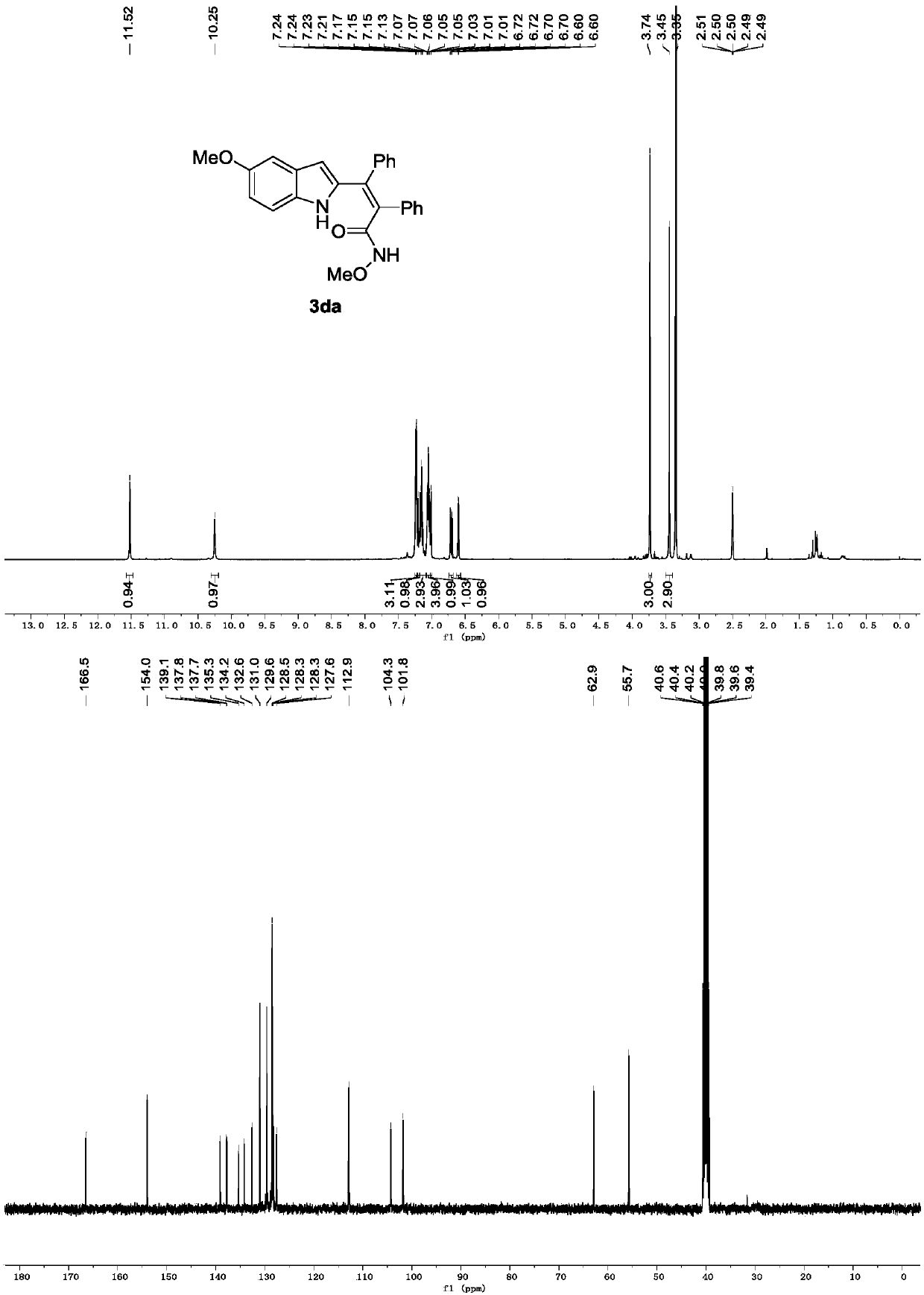
[0032] Replace 1a in Example 1 with 1c, and other conditions are the same as Example 1. The experimental results are shown in Table 1.
[0033]
[0034] Spectrum analysis data 3ca:
[0035] 1 H NMR (400MHz, DMSO-d 6 )δ11.56(s,1H),10.67(s,1H),7.59(d,J=2.0Hz,1H),7.33(d,J=8.6Hz,1H),7.25(dd,J=5.0,1.7 Hz,3H),7.19(s,1H),7.16(d,J=2.6Hz,2H),7.05(ddt,J=7.4,3.3,1.7Hz,5H),6.68(d,J=1.5Hz,1H ),3.42(s,3H). 13 C NMR (101MHz, DMSO-d 6 ( 24 h 19 ClN 2 NaO 2 ++ [M+Na] + 425.1033, found 425.1028.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com