Anthracene nitrogen-containing organic light-emitting compound and preparation method and application thereof
A luminescent compound, an organic technology, applied in the direction of organic chemistry, chemical instruments and methods, luminescent materials, etc., can solve different problems and achieve the effects of avoiding aggregation, good electron transport performance, thermal stability and chemical stability improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
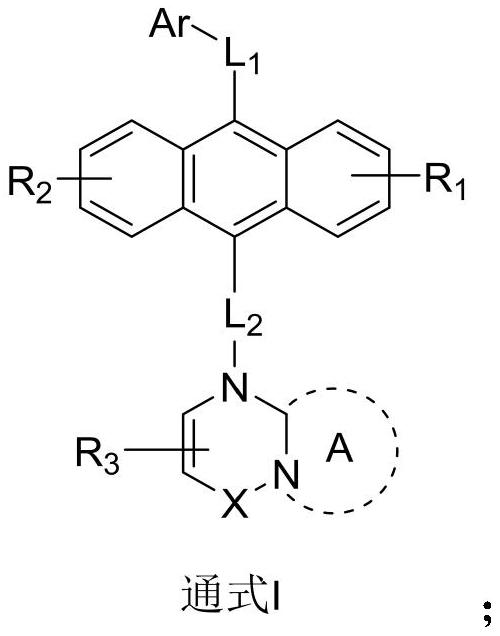
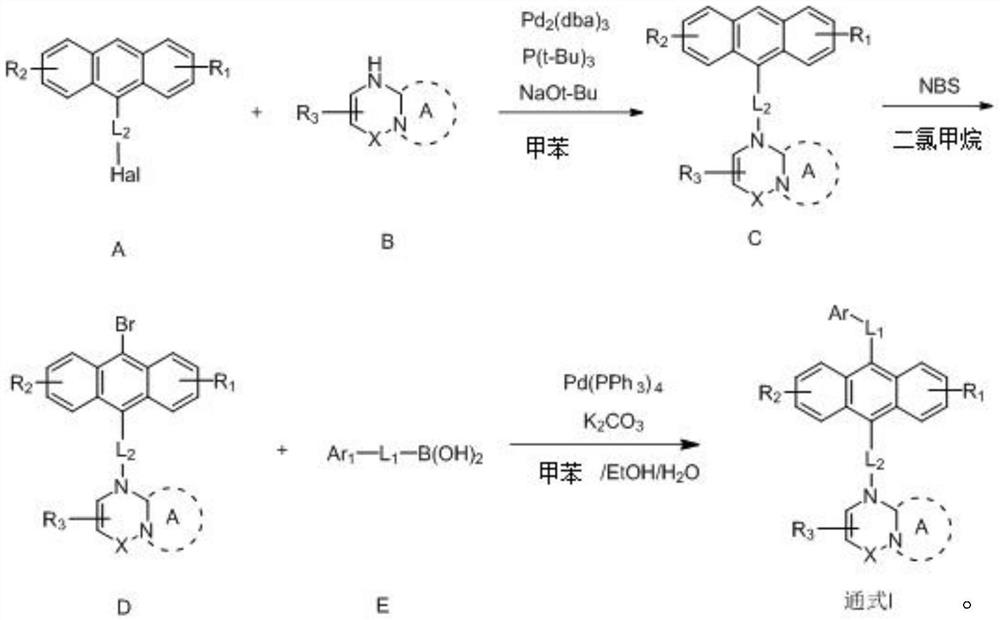
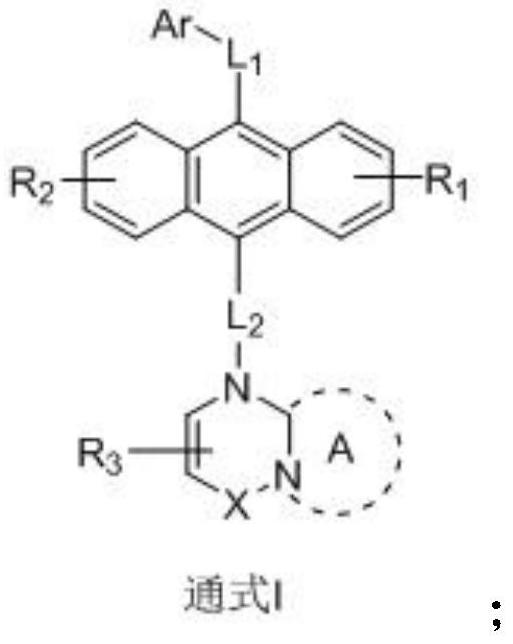
[0055] The embodiment of the present invention also provides a preparation method of an anthracene nitrogen-containing organic light-emitting compound, which is characterized in that it includes the following steps:
[0056] 1) Dissolving raw material A, raw material B and sodium tert-butoxide in toluene under the protection of an inert gas to obtain a mixture 1;
[0057] 2) Add tris(dibenzylideneacetone)dipalladium and tris(tert-butyl)phosphorus into mixture 1, and react under reflux and stirring at 100-120°C for 12 hours to obtain mixture 2;
[0058] 3) After removing impurities, washing, and extracting the mixture 2, the organic phase was combined, and then the organic phase was dried with anhydrous magnesium sulfate, filtered to remove the anhydrous magnesium sulfate, concentrated and purified to obtain an intermediate product C;
[0059] 4) At room temperature, add NBS to the dichloromethane solution of the intermediate product C to react. After the reaction is completed,...
Embodiment 1
[0070] A preparation of an anthracene nitrogen-containing organic light-emitting compound (compound 1), its specific synthesis steps are as follows:
[0071]
[0072] 1) Under nitrogen protection, raw material A-1 (50 mmol), raw material B-1 (55 mmol) and sodium tert-butoxide (150 mmol) were put into a flask and dissolved in dry toluene (200 mL). Then, Pd 2 (dba) 3 (0.56mmol) and P(t-Bu) 3 (2.8 mmol) was added to the above reactant, heated to 110° C., refluxed and stirred for 12 hours. After the reaction, diatomaceous earth was used to remove the salt and the catalyst, washed with water, and the retained organic phase was extracted with dichloromethane. The organic phases were combined, and the extract was dried over anhydrous magnesium sulfate and filtered. Then, the filtered product was concentrated under reduced pressure. And the solvent was removed using a rotary evaporator. Intermediate C-1 (23.3 g, 85% yield, Ms: 549.22) was obtained.
[0073] 2) Intermediate C...
Embodiment 2
[0077] A preparation of an anthracene nitrogen-containing organic light-emitting compound (compound 20), the specific synthesis steps are as follows:
[0078]
[0079] 1) Under nitrogen protection, raw material A-20 (50 mmol), raw material B-20 (55 mmol) and sodium tert-butoxide (150 mmol) were put into a flask and dissolved in dry toluene (200 mL). Then, the three Pd 2 (dba) 3 (0.56mmol) and P(t-Bu) 3 (2.8 mmol) was added to the above reactant, the temperature was raised to 110° C., then refluxed and stirred for 12 hours. After the reaction, diatomaceous earth was used to remove the salt and the catalyst, washed with water, and the retained organic phase was extracted with dichloromethane. The organic phases were combined, and the extract was dried over anhydrous magnesium sulfate and filtered. Then, the filtered product was concentrated under reduced pressure. And the solvent was removed using a rotary evaporator. Intermediate C-20 (25.6 g, 82% yield, Ms: 624.79) wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com