Photoelectric function material with N-P=S resonant structure, preparation method and application
A technology of optoelectronic functional materials and resonant structures, which is applied in the fields of luminescent materials, chemical instruments and methods, circuits, etc., can solve the problems of high concentration quenching, reduced device efficiency, triplet-triplet annihilation, etc., and achieves low cost and low cost. Start-up voltage, enhanced transfer and recombination effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
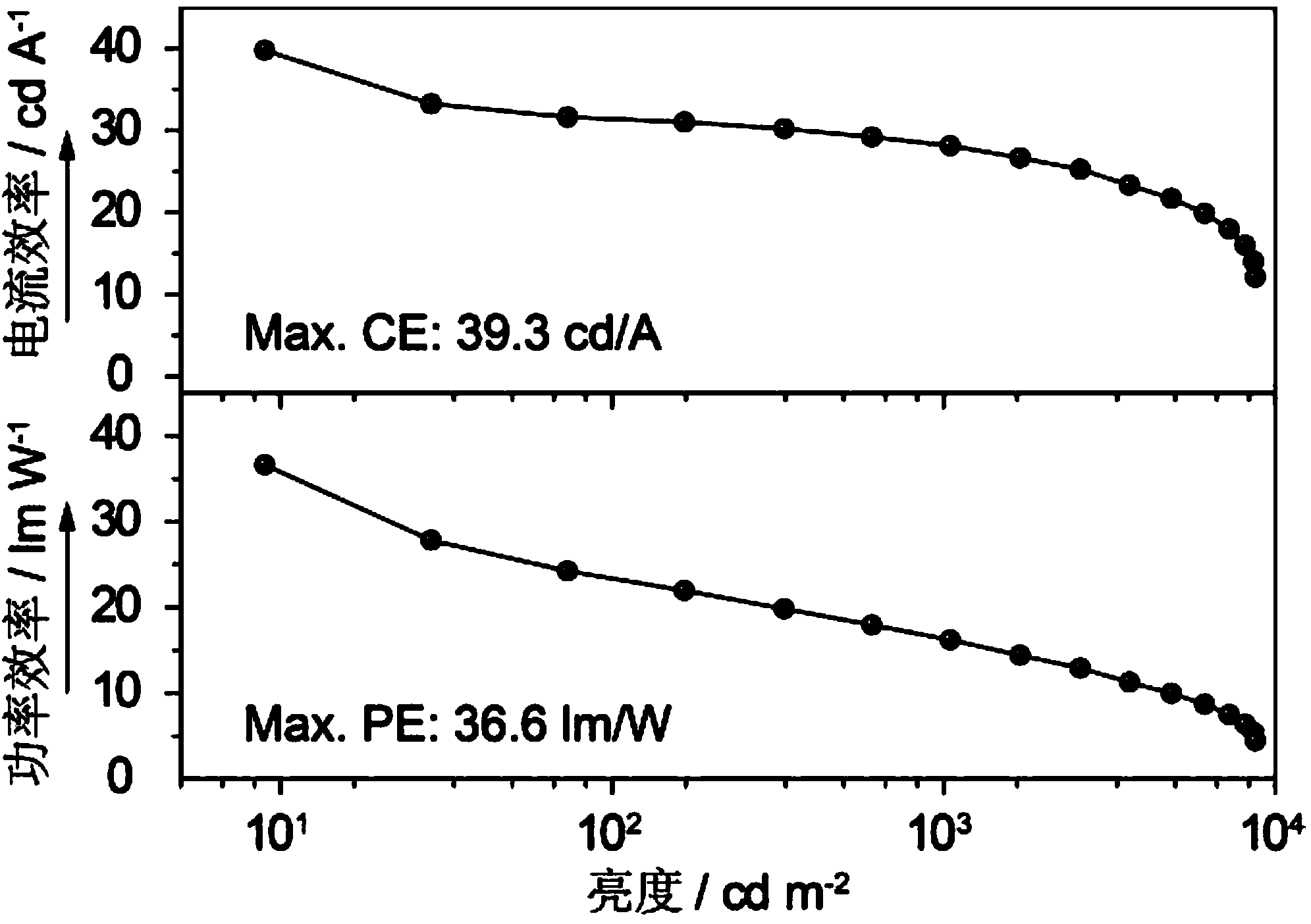
Examples
Embodiment 1
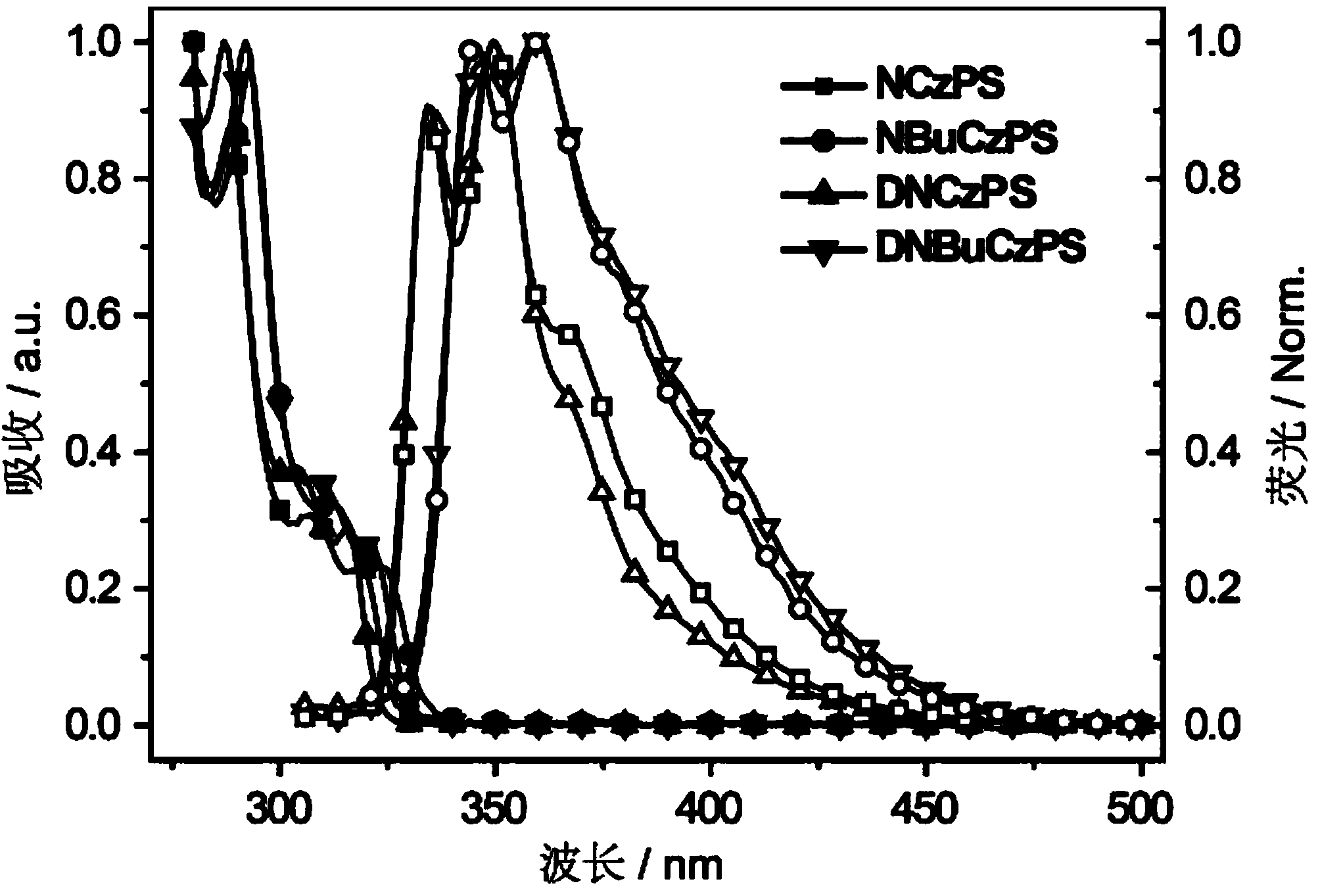
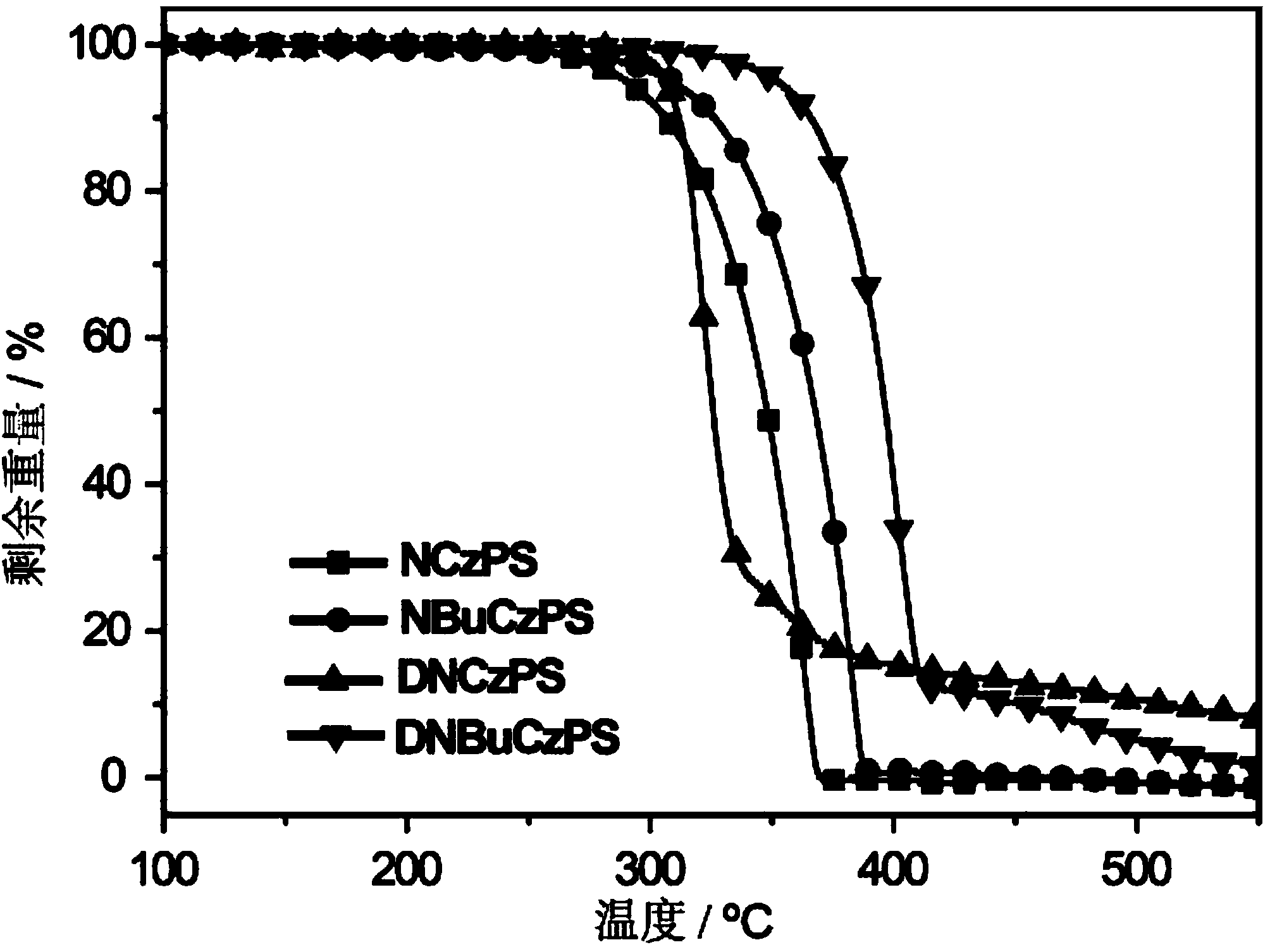
[0034] Embodiment 1: Synthesis of photoelectric functional material NCzPS
[0035] Add 1.67g carbazole to the reaction bottle, vacuumize, blow nitrogen repeatedly three times, add 20ml of anhydrous tetrahydrofuran (THF) under nitrogen protection, and then put the device into a dry ice / acetone bath at -78°C to cool for 10min. Measure 7.5ml of n-butyllithium n-hexane solution with a syringe, add it dropwise into the reaction flask, react at -78°C for 1h under nitrogen protection, then add 2.3ml of diphenylphosphorus chloride, and react at room temperature for 8h. The reaction solution was quenched with 10ml of water, extracted with 3×30mL of dichloromethane solution, and the organic phase was collected and dried over anhydrous sodium sulfate. The dichloromethane solution was spun out with a rotary evaporator. Then the crude product was dissolved in 50ml of dichloromethane, and 0.32g of sulfur element was added and stirred for 8h. Extract with 3×30mL solution, collect the organ...
Embodiment 2
[0037] Embodiment 2: the synthesis of photoelectric functional material NBuCzPS
[0038]Add 2.79g of tert-butyl carbazole to the reaction bottle, vacuumize, blow nitrogen repeatedly three times, add 20ml of anhydrous tetrahydrofuran (THF) under nitrogen protection, and then put the device into a dry ice / acetone bath at -78°C to cool for 10min . Measure 7.5ml of n-butyllithium n-hexane solution with a syringe, add it dropwise into the reaction flask, react at -78°C for 1h under nitrogen protection, then add 2.3ml of diphenylphosphorus chloride, and react at room temperature for 8h. The reaction solution was quenched with 10ml of water, extracted with 3×30mL of dichloromethane solution, and the organic phase was collected and dried over anhydrous sodium sulfate. The dichloromethane solution was spun out with a rotary evaporator. Then the crude product was dissolved in 50ml of dichloromethane, and 0.32g of sulfur element was added and stirred for 8h. Extract with 3×30mL soluti...
Embodiment 3
[0040] Embodiment 3: Synthesis of photoelectric functional material DNCzPS
[0041] Add 1.0 g of carbazole to the reaction flask, vacuumize and blow nitrogen three times, add 10 ml of anhydrous tetrahydrofuran (THF) under nitrogen protection, and then place the device in a dry ice / acetone bath at -78°C for 10 minutes to cool. Use a syringe to measure 4.5ml of 1.6M n-butyllithium n-hexane solution, add dropwise to the reaction flask, react at -78°C for 1h under nitrogen protection, then add 0.41ml of phenylphosphorous dichloride, and then react at room temperature for 8h . The reaction solution was quenched with 10ml of water, extracted with 3×30mL of dichloromethane solution, and the organic phase was collected and dried over anhydrous sodium sulfate. The dichloromethane solution was spun out with a rotary evaporator. Then the crude product was dissolved in 50ml of dichloromethane, and 0.1g of sulfur was added and stirred for 8h. Extract with 3×30mL solution, collect the or...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com