Copper fluoride (I) reagent as well as preparation method and application thereof
A technology of copper fluoride and reagents, which is applied in the field of catalyst synthesis, can solve problems such as difficulty in popularization and instability, and achieve good application prospects, easy operation, and good reactivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] In a 50ml one-necked flask, add a polytetrafluoroethylene magnetic stirring bar, and weigh 0.21mmol sodium tert-butoxide, 0.20mmol cuprous chloride, add 8ml acetonitrile solvent and mix well, at room temperature and under the protection of nitrogen, react After one hour, the solution was filtered, and 0.20 mmol of 2,9-dimethyl-1,10-phenanthroline in tetrahydrofuran was added to the filtrate. After the solution turned reddish-brown, it was stirred for 5 minutes, and 0.20 mmol of triethylamine trihydrogen trifluoride was added dropwise. Stir for 5 minutes, filter the solution, wash the solid with n-hexane, and drain it to obtain 48 mg (92%) of an orange-red solid, which is the complex of 2,9-dimethyl-1,10-phenanthroline-copper(I) fluoride things.
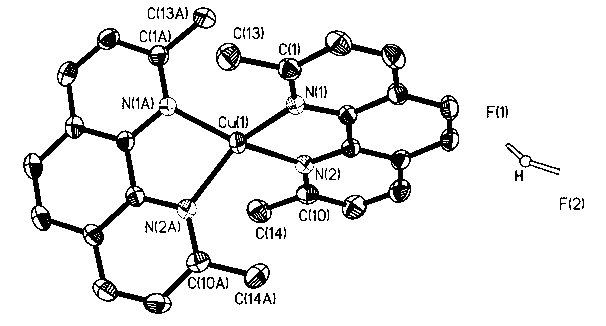
[0016] Attached picture 1 is a single crystal structure, where 1 H NMR (DMSO-d 6 ): 8.76 (d, 2H, J = 8.22Hz), 8.22 (s, 2H), 7.96 (d, 2H, J = 8.22Hz), 2.39 (s, 6H). 19 F NMR (DMSO-d 6 ): 3.35. 13 C NMR (DMSO-d 6 ): 15...
Embodiment 2
[0018] In a 50ml one-necked flask, add a polytetrafluoroethylene magnetic stirring bar, and weigh 0.21mmol sodium tert-butoxide, 0.20mmol cuprous chloride, add 8ml acetonitrile solvent and mix well, at room temperature and under the protection of nitrogen, react One hour, filter the solution, add 0.20mmol 2,9-di-tert-butyl-1,10-phenanthroline tetrahydrofuran solution to the filtrate, the solution turns reddish brown, then stir for 5min, add dropwise 0.20mmol triethylamine trihydrogen fluoride , stirred for 5 min, the solution was filtered, the filtrate was desolventized in vacuo, the solid was dissolved in toluene, covered with n-hexane, and recrystallized at -10°C to obtain 29 mg (38%) of orange-red crystals, which were 2,9-di-tert-butyl- 1,10-Phenanthroline Copper(I) Fluoride Complex.
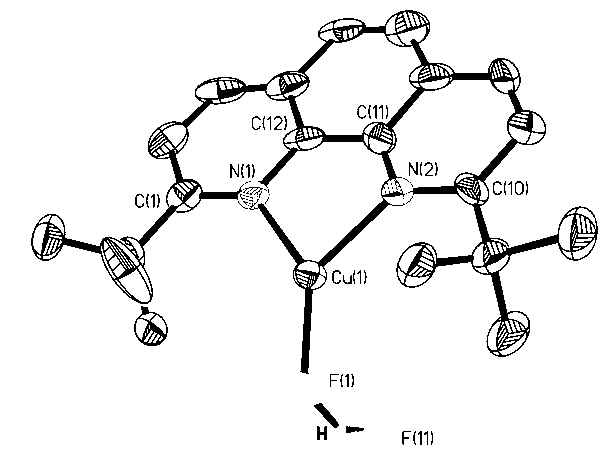
[0019] Attached picture 2 is a single crystal structure, where 1 H NMR (DMSO-d 6 ): 8.46 (d, 2H, J = 8.52 Hz), 8.05 (s, 2H), 7.98 (d, 2H, J = 2.31Hz), 1.60 (s, 18H). 19 F NMR (DMSO-d 6 )...
Embodiment 3
[0021] The 2,9-dimethyl-1,10-phenanthroline compound copper fluoride (I) reagent prepared by the method of Example 1, in a nitrogen atmosphere, put a polytetrafluoroethylene magnet in a reactor , add 0.025mmol 2,9-dimethyl-1,10-phenanthroline copper (I) fluoride reagent ((Me 2 phen) 2 Cu(HF 2 )) and 0.125mmol 1-bromo-3-phenylpropane, add 1ml of acetonitrile solvent, heat in a closed system at 110°C for 15h, cool to room temperature, add 10uL trifluoromethoxybenzene to the reaction solution, and measure the obtained Product of 1-fluoro-3-phenylpropane 19 F NMR yield, ( 19 F NMR yield 92%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com