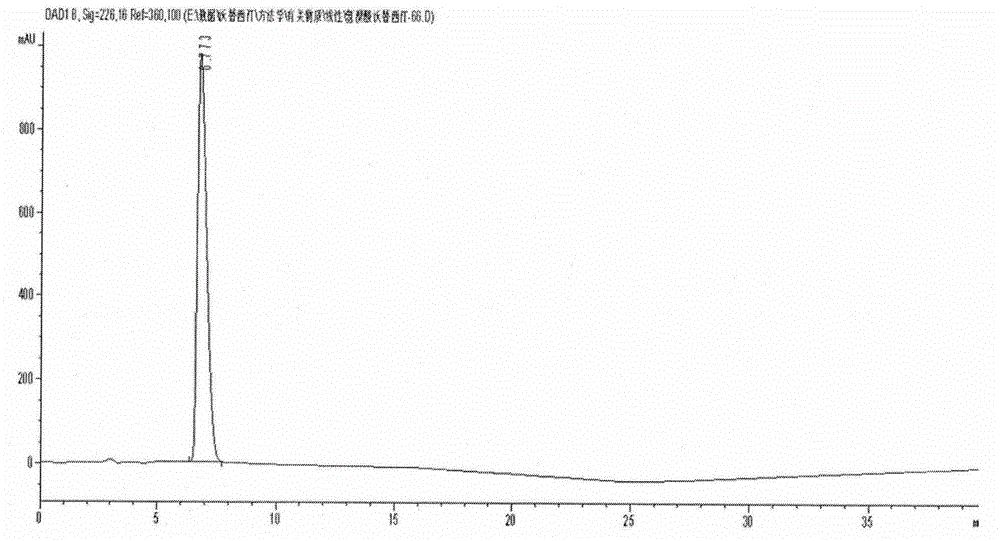
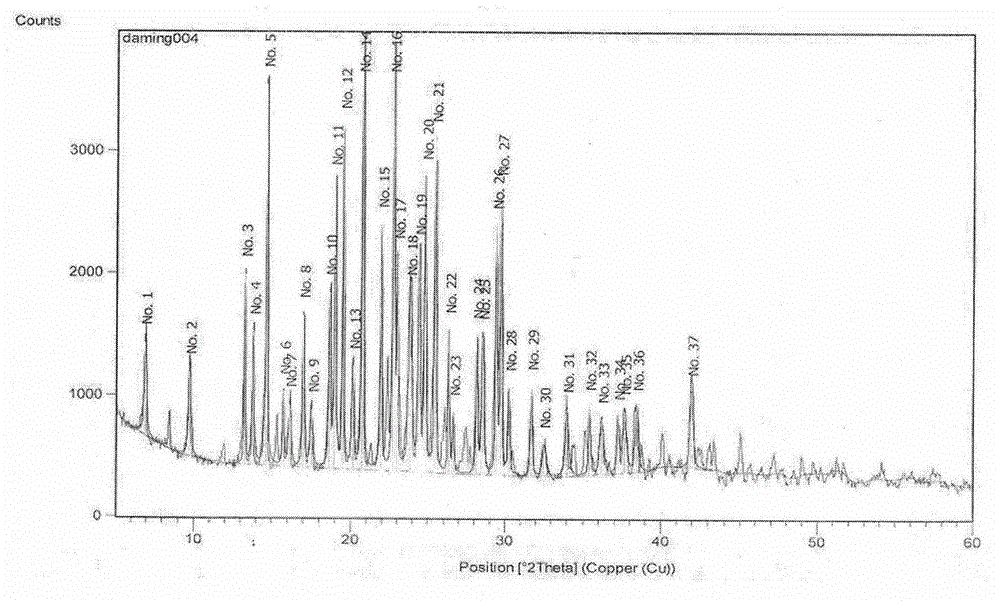
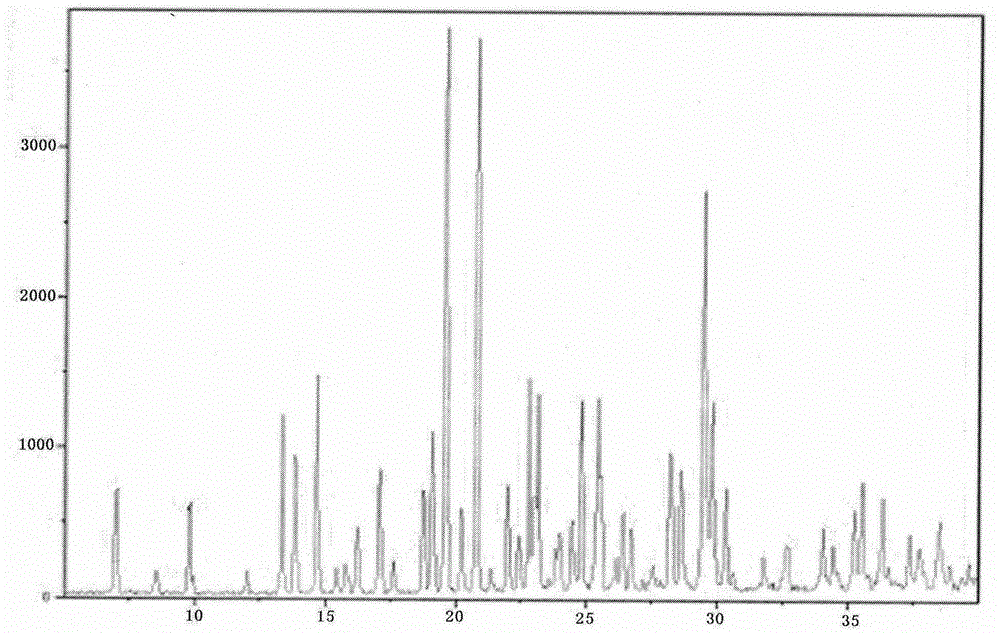
Novel preparation method of hydrobromic acid Vortioxetine beta crystalline form
A technology of vortioxetine hydrobromide and vortioxetine, which is applied in the field of drug synthesis, can solve the problems of high purity of vortioxetine hydrobromide, low purity of vortioxetine, high cost, etc. The effect of industrialized production, high product yield and mild process reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: This example provides a new method for preparing vortioxetine hydrobromide β crystal form. The specific steps are as follows:
[0025] Step 1: Synthesis of 2-(2,4-dimethylphenylsulfanyl)chlorobenzene
[0026]
[0027] Take a 250ml reaction flask, pour nitrogen into the flask, take 20g (0.14mol) of 2-chlorothiophenol (formula I) and 17.1g (0.14mol) of 2,4-dimethylphenol (formula II) into the reaction Add 153g of ethyl acetate, 0.8g (0.014mol) of nickel nanopowder, 34.4g (0.42mol) of sodium isopropoxide and 10g of anhydrous sodium sulfate into the bottle, stir at room temperature for 20min, warm up to 50℃, and stir for 8h. TLC monitors the progress of the reaction. After the reaction is over, the heating is stopped. After the reaction solution is cooled to room temperature, it is filtered. The filtrate is washed 3 times with 50ml each time. The organic phase is taken and dried with anhydrous sodium sulfate and filtered. The solvent was evaporated under a vacuum of ...
Embodiment 2
[0035] Example 2: This example provides another new method for preparing vortioxetine hydrobromide β crystal form. The specific steps are as follows:
[0036] Step 1: Synthesis of 2-(2,4-dimethylphenylsulfanyl)chlorobenzene
[0037]
[0038] Take a 500ml reaction flask, pour nitrogen into the flask, take 40g (0.28mol) of 2-chlorothiophenol (formula I) and 34.2g (0.28mol) of 2,4-dimethylphenol (formula II) into the reaction Add 306g ethyl acetate, 1.58g (0.027mol) of nickel nanopowder, 71.1g (0.87mol) of sodium isopropoxide and 23.7g of anhydrous sodium sulfate into the bottle, stir at room temperature for 30min, warm up to 60℃, and stir for 9h , TLC monitors the progress of the reaction. After the reaction is over, stop heating, filter the reaction solution after it has cooled to room temperature (20~25℃), wash the filtrate 4 times with 50ml each time, take the organic phase and dry it with anhydrous sodium sulfate overnight , Filter, and use a rotary evaporator to distill off the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com