Synthesis method of succinic acid derivative or 3-aryl propionic acid
An arylpropionic acid and a synthesis method technology, applied in the directions of organic chemistry methods, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of low atom economy, excessive oxidation, complicated operation, etc., and achieve good industrial application Prospects, mild reaction conditions, cheap and readily available raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment one: Synthesis of succinic acid derivatives
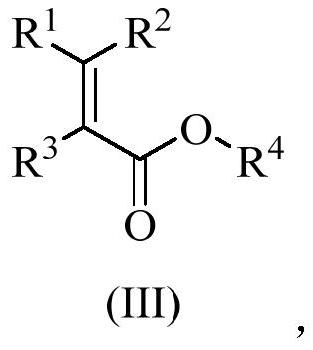
[0049] The synthetic reaction formula of succinic acid derivative is shown in formula (III),
[0050]
[0051] The synthesis of succinic acid derivatives comprises the following steps:
[0052] S1: Transfer the dry Schlenk tube (10 mL) with magnets into the glove box, add sodium tert-butoxide (48 mg, 0.5 mmol, 2.5 times equivalent);
[0053] S2: Take the Schlenk tube out of the glove box and connect it to the 2 On the double-row tube of the steel cylinder, under the condition that the Schlenk tube is closed, the double-row tube is pumped with CO 2 At least 3 times, excluding N in branch 2 , so that it is filled with CO 2 gas;
[0054] S3: in CO 2 NMP (2 mL), tert-butanol (38 μL, 0.4 mmol, 2 equivalents), 2,4,6-triisopropylthiophenol (95 mg, 0.4 mmol, 2 equivalents) and the reaction substrate ( 1) (0.2mmol);
[0055] S4: Seal the Schlenk tube and place it under liquid nitrogen. After it is completely fro...
Embodiment 2
[0101] Embodiment two: Synthesis of 3-aryl propionic acid
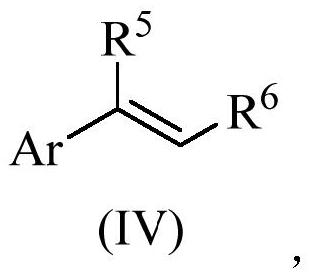
[0102] The synthetic reaction formula of 3-aryl propionic acid is shown in formula (II),
[0103]
[0104] The synthesis of 3-aryl propionic acid comprises the following steps:
[0105]S1: Transfer the dry Schlenk tube (10 mL) with the magneton into the glove box, add sodium tert-butoxide (48 mg, 0.5 mmol, 2.5 times equivalent);
[0106] S2: Take the Schlenk tube out of the glove box and connect it to the 2 On the double-row tube of the steel cylinder, under the condition that the Schlenk tube is closed, the double-row tube is pumped with CO 2 At least 3 times, excluding N in branch 2 , so that it is filled with CO 2 gas;
[0107] S3: in CO 2 Add NMP (2 mL), tert-butanol (38 μL, 0.4 mmol, 2 equivalents), 4-tert-butylthiophenol (66.5 mg, 0.4 mmol, 2 equivalents) and reaction substrate (3) (0.2 mmol);
[0108] S4: Seal the Schlenk tube and place it under liquid nitrogen. After it is completely frozen, open th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com