Preparation method of Venetoclax intermediate and product
A technology of venetola and an intermediate, which is applied in the field of medicine, can solve the problems of unsuitability for large-scale production, low total yield, long steps and the like, and achieves a technology that is beneficial to large-scale industrial production, reduces production costs and consumes low energy. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
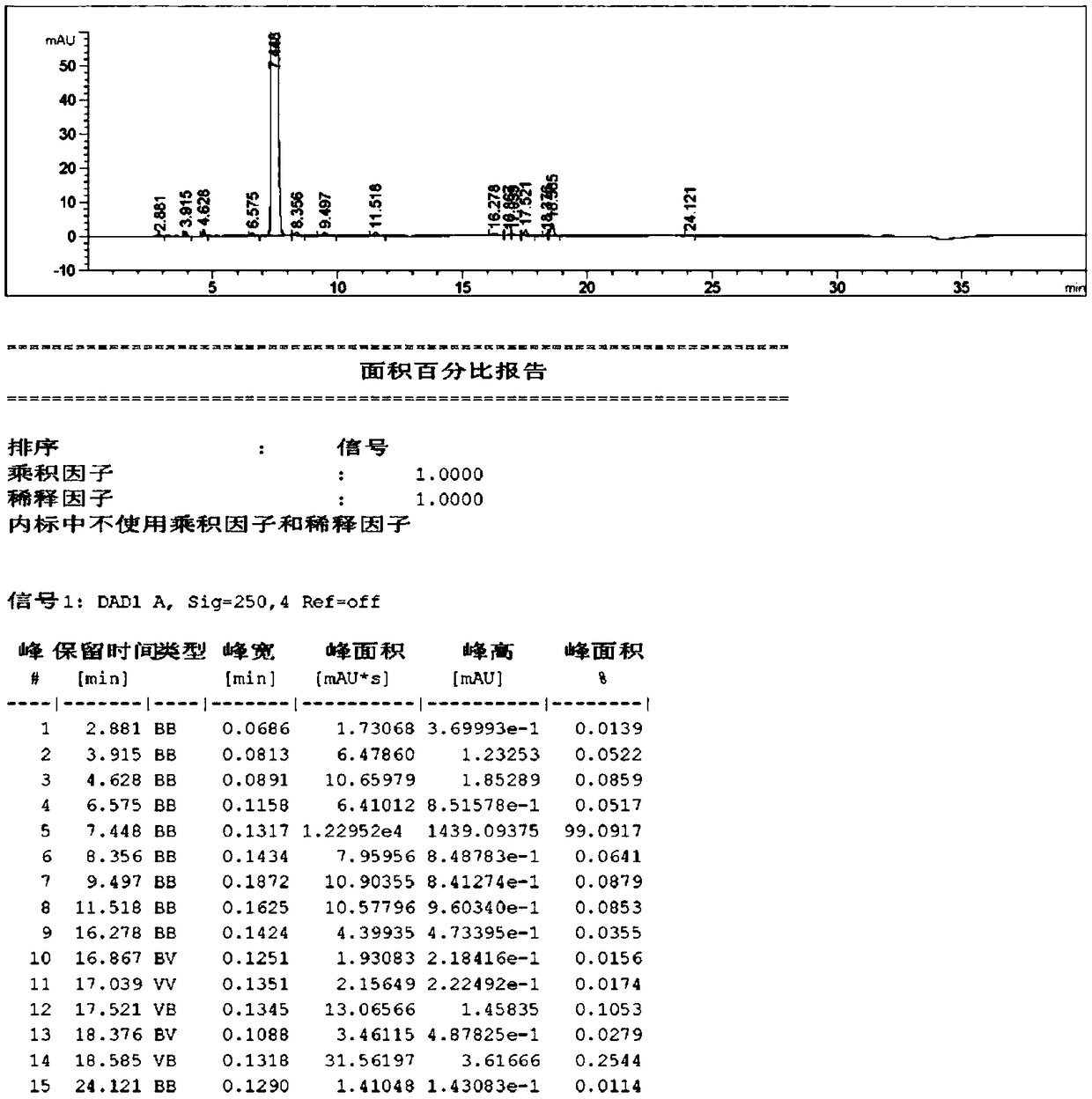
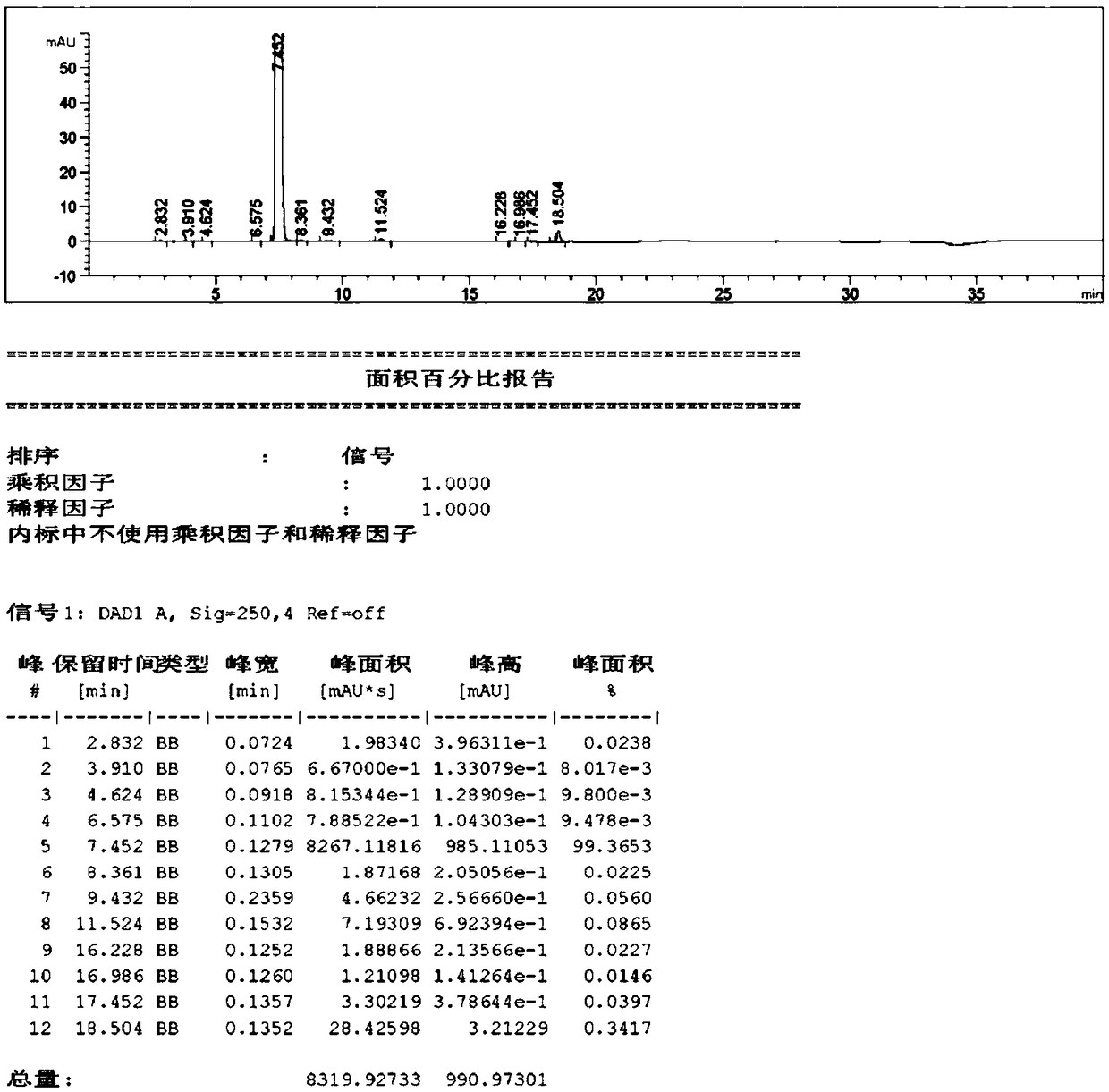
Image
Examples
Embodiment 1
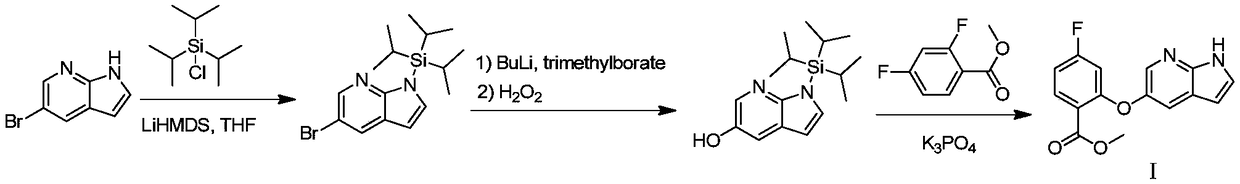
[0041] Step 1: Preparation of 5-bromo-1-(triisopropylsilyl)-1H-pyrrolo[2,3-b]pyridine (Ⅲ)
[0042]
[0043] Dissolve 5-bromo-7-azaindole (20.0g, 101.5mmol) in 200mL tetrahydrofuran, add potassium tert-butoxide (13.7g, 121.8mmol) at room temperature, raise the temperature to 60-65°C, and stir for 2 hours. The temperature was lowered to 10-20°C, and triisopropylchlorosilane (21.5g, 111.7mmol) was added dropwise. After the dropwise addition, the reaction was continued at 10-20°C for 4 hours. TLC detection showed that the reaction of the raw materials was complete. After the reaction is complete, add 100 mL of water to quench, extract once with 200 mL of ethyl acetate, wash the extract once with 200 mL of saturated brine, dry over anhydrous sodium sulfate, filter, and spin dry, the crude product is recrystallized with 200 mL of methanol, filtered, and dried to obtain Light yellow powder 30.9g, yield 86.3%.
[0044] Step 2: Preparation of 1-(triisopropylsilyl)-1H-pyrrolo[2,3-b]...
Embodiment 2
[0051] Step 1: Preparation of 5-bromo-1-(triisopropylsilyl)-1H-pyrrolo[2,3-b]pyridine (Ⅲ)
[0052]
[0053] Dissolve 5-bromo-7-azaindole (100.0 g, 0.51 mol) in 200 mL of tetrahydrofuran, add potassium tert-butoxide (68.5 g, 0.61 mol) at room temperature, raise the temperature to 60-65 °C, and stir for 2 hours. The temperature was lowered to 10-20°C, and 107.5 g (0.56 mol) of triisopropylchlorosilane was added dropwise. After the dropwise addition, the reaction was continued at 10-20°C for 4 hours. TLC detection showed that the reaction of the raw materials was complete. After the reaction was completed, 500 mL of water was added to quench, extracted once with 1000 mL of ethyl acetate, the extract was washed once with 1000 mL of saturated brine, dried over anhydrous sodium sulfate, filtered, and spin-dried, the crude product was recrystallized with 1000 mL of methanol, filtered, and dried Yellow powder 156.7g, yield 87.5%.
[0054] Step 2: Preparation of 1-(triisopropylsily...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com