Preparation method of cloperadine hydrochloride
A technology of cloperidine hydrochloride and salt formation, applied in the field of medicine, can solve the problems of reduced yield of target product, reduced utilization rate of chloroethanol, increased production cost of enterprises, etc., and is beneficial to large-scale industrial production, reduced extraction cost, and improved quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
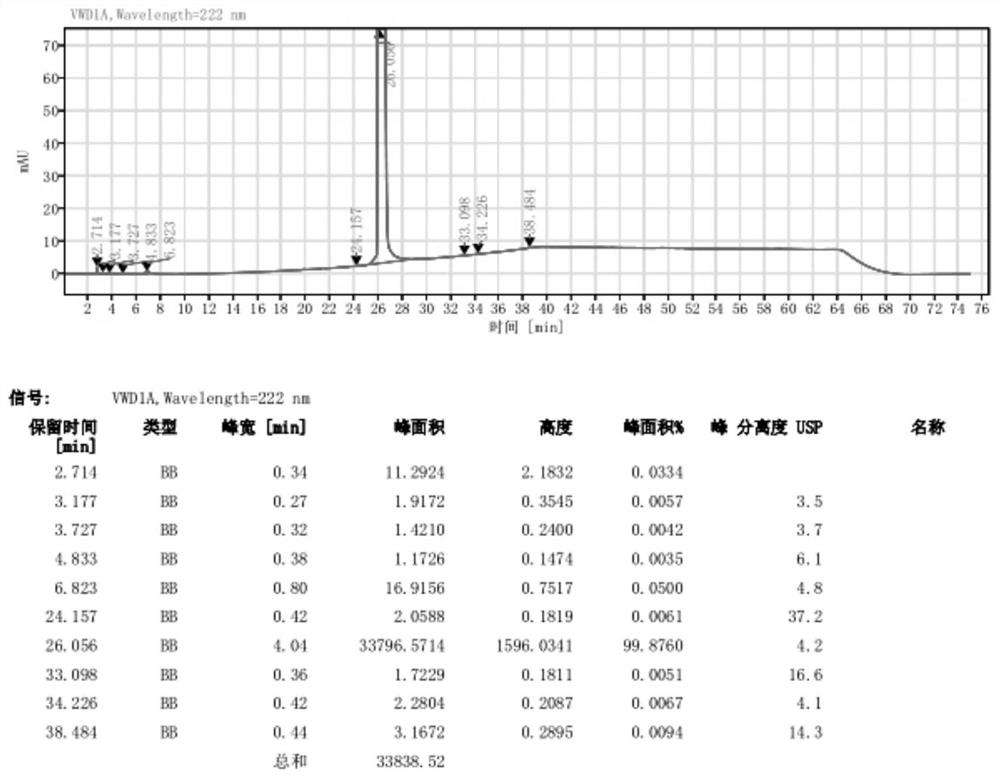
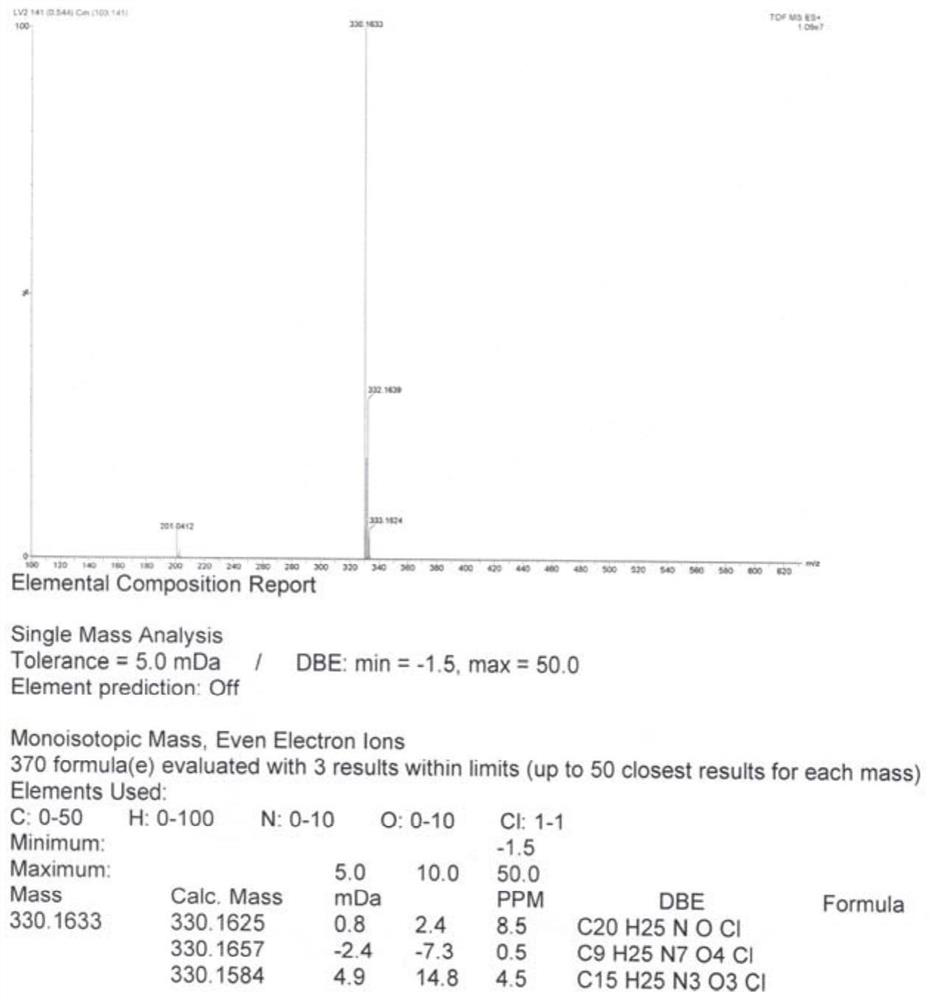
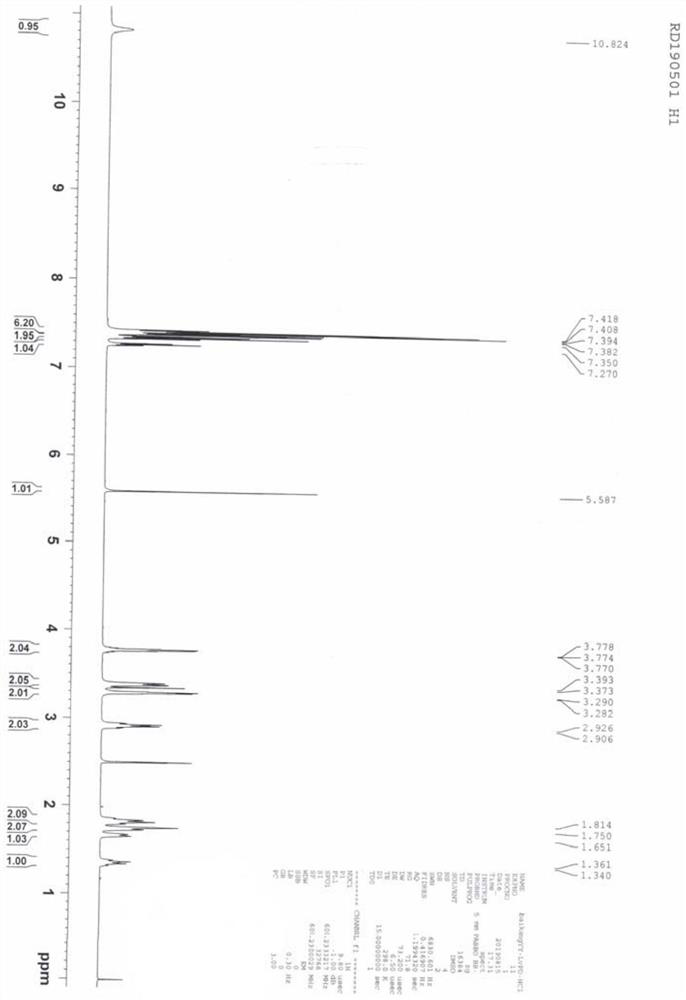
Image
Examples
Embodiment 1
[0039] Step 1: Add 20g (84.7mmol) of 4-chlorodiphenylchloromethane, 10.9g (84.7mmol) of 2-piperidineethanol and 80mL of dichloromethane to the three-necked reaction flask in sequence, and add 10.3g of triethylamine under stirring (101 mmol), then reacted at 20-30° C. for 3 hours, and TLC detected that the reaction of the raw materials was complete. The reaction solution was washed twice with water, and the organic phase was spin-dried to obtain compound (II) as an oil, which was directly used in the next step.
[0040] Step 2: Dissolve the oily substance obtained in the previous step in 60 mL of isopropyl acetate, feed hydrogen chloride gas at 20-30°C until the pH is less than 2, then stop stirring at 20-30°C for 1 hour, then cool down to 0-30°C Stir at 5°C for 1 hour, filter with suction, wash the filter cake with isopropyl acetate, and dry the wet product under reduced pressure to obtain 28.2 g of white powder with a yield of 91.2%, a purity of 99.8%, and a maximum single im...
Embodiment 2
[0042] Step 1: Add 20g (91.7mmol) of 4-chlorodiphenylmethanol, 13.5g (91.7mmol) of N-(2-chloroethyl)piperidine, and 0.2g of tetrabutylammonium iodide to the three-necked reaction flask in sequence and 80 mL of dichloromethane, and added 11.2 g (111 mmol) of triethylamine under stirring, and then reacted at 30-40° C. for 5 hours, and the reaction of raw materials was detected by TLC. The temperature was lowered to 30°C, washed with water twice and spin-dried to obtain compound (II) as an oil, which was directly used in the next step.
[0043] Step 2: Dissolve the oily substance obtained in the previous step in 60 mL of ethyl acetate, feed hydrogen chloride gas at 20-30°C until the pH is less than 2, stop, continue to stir at 20-30°C for 1 hour, and then cool down to 0-5 Stir at ℃ for 1 hour, filter with suction, wash the filter cake with ethyl acetate, and dry the wet product under reduced pressure to obtain 30.1 g of white powder with a yield of 89.8%, a purity of 99.8%, and a...
Embodiment 3
[0045]Step 1: In the three-necked reaction flask, add 4-chlorodiphenylmethanol 20g (91.7mmol), 2-piperidineethanol 11.8g, 13.5g (91.7mmol) and 80mL toluene in sequence, and add concentrated sulfuric acid 5.4g ( 55.1 mmol), and then reacted at 100-110° C. for 7 hours, and TLC detected that the reaction of the raw materials was complete. Cool down to 0-5°C to crystallize for 1 hour, filter with suction, adjust the pH to alkaline with lye (sodium hydroxide, potassium hydroxide, carbonate or bicarbonate), add 60 mL of ethyl acetate to extract, organic The phase was washed once and directly used in the next step for salt formation.
[0046] Step 2: Add hydrogen chloride gas to the ethyl acetate compound (II) solution obtained in the previous step at 20-30°C until the pH is less than 2, then stop, continue to stir at 20-30°C for 1 hour, and then cool down to 0-5°C Stir for 1 hour, filter with suction, wash the filter cake with isoethyl acetate, and dry the wet product under reduced...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com